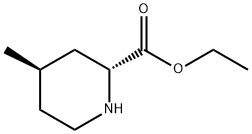
Ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate synthesis
- Product Name:Ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate
- CAS Number:74892-82-3
- Molecular formula:C9H17NO2
- Molecular Weight:171.24
![2-Piperidinecarboxylic acid, 4-methyl-1-[(1S)-1-phenylethyl]-, ethyl ester, (2R,4R)-](/CAS/20210305/GIF/198641-56-4.gif)
198641-56-4
0 suppliers
inquiry

74892-82-3
223 suppliers
$80.00/500mg
Yield:74892-82-3 345 g
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen;iron(II) oxalate;acetic acid in ethanol at 30; under 3750.38 Torr; for 4 h;Autoclave;Reagent/catalyst;
Steps:
1.2 two,(2R, 4R) -4-methylpiperidine-2-carboxylate Preparation
The crude product (618 g) obtained in the previous step and ethanol (2 mL) were charged into a 5 L autoclave,Additional acetic acid (45 g), palladium on carbon catalyst (10% palladium loading, 15 g), 3 g iron oxalate,Access to H2, at 30 , 0.5MPa reaction 4h,filter,Catalyst recovery.The filtrate was concentrated under reduced pressure and added with ethyl acetate (1 L)Washed sequentially with saturated sodium carbonate solution (250 mL × 2), saturated brine (250 mL × 2)Dried over anhydrous sodium sulfate, filtered,The filtrate was concentrated under reduced pressure,Have a pale yellow liquid product.The resulting crude product was purified by distillation,Collected 89 ~ 90 / 10mmHg fractions,345 g of ethyl (2R, 4R) -4-methyl-2-piperidinecarboxylate was obtained in a yield of 91%.Optical rotation: (c = 5, EtOH)The total process yield above: 82.45%.Liquid chromatography determination:The (2R, 4R) -4-methyl-2-piperidinecarboxylic acid ethyl ester content was 99.3%.
References:
CN107043347,2017,A Location in patent:Paragraph 0029; 0030; 0031; 0032-0034; 0039-0044; 0046; 0048
131278-84-7
12 suppliers
inquiry

74892-82-3
223 suppliers
$80.00/500mg
![(2R)-1,2,3,6-Tetrahydro-4-Methyl-1-[(1R)-1-phenylethyl]-2-pyridinecarboxylic Acid Ethyl Ester](/CAS/20150408/GIF/139334-62-6.gif)
139334-62-6
5 suppliers
inquiry

74892-82-3
223 suppliers
$80.00/500mg
78-79-5
261 suppliers
$14.56/25ML

74892-82-3
223 suppliers
$80.00/500mg
139334-63-7
1 suppliers
inquiry

74892-82-3
223 suppliers
$80.00/500mg