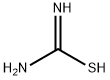
Ethyl 2-bromothiazole-4-carboxylate synthesis
- Product Name:Ethyl 2-bromothiazole-4-carboxylate
- CAS Number:100367-77-9
- Molecular formula:C6H6BrNO2S
- Molecular Weight:236.09
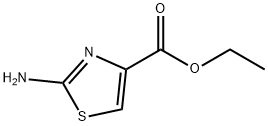
5398-36-7
393 suppliers
$6.00/5g

100367-77-9
256 suppliers
$5.00/250mg
Yield:100367-77-9 84%
Reaction Conditions:
with 2-Methyl-2-nitropropane;copper(ll) bromide in acetonitrile at 0 - 20; for 12 h;
Steps:
1.A Step A: Preparation of ethyl 2-bromo-1,3-thiazole-4-carboxylate
To a solution of ethyl 2-aminothiazole-4-carboxylate (100 g, 581 mmol) and copper (II) bromide (195 g,871 mmol) in acetonitrile (1000 ml) at 0 °C, tert-butylnitrite (104 ml, 871 mmol) was added dropwise. The reaction mixture was warmed to room temperature and stirred for 12h. After completion of thereaction, the reaction mixture was diluted with a mixture of ethyl acetate (1000 ml) and water (3000 ml) and then acidified to pH 2 using iN hydrochloric acid. The two layers were separated and the aqueous layer was again extracted three times with ethyl acetate (500 ml). The combined organic layers were dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by recrystallization from hexane to obtain pure ethyl 2-bromo-1,3-thiazole-4-carboxylate (115 g,84% yield).‘H-NMR (400 MHz, DMSO-d6) 8.52 (s, IH), 4.29 (q, J 7.1 Hz, 2H), 1.29 (t,J 7.1 Hz, 3H)MS: m/z235.90. [M+1].
References:
PI INDUSTRIES LTD.;SHANBHAG, Gajanan;DODDA, Ranga Prasad;KAMBLE, Ganesh Tatya;KALE, Yuvraj Navanath;RENUGADEVI, G.;MANJUNATHA, Sulur G;S.P., Mohan Kumar;AUTKAR, Santosh Shridhar;GARG, Ruchi;VENKATESHA, Hagalavadi M;MAVINAHALLI, Jagadeesh Nanjegowda;KLAUSENER, Alexander G.M. WO2018/193387, 2018, A1 Location in patent:Page/Page column 92

70-23-5
315 suppliers
$6.00/5g

100367-77-9
256 suppliers
$5.00/250mg
56417-52-8
4 suppliers
inquiry

75-34-3
106 suppliers
$22.69/48602
7789-60-8
387 suppliers
$10.40/25G

100367-77-9
256 suppliers
$5.00/250mg