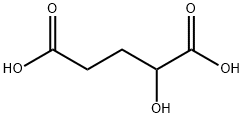
DL-Glutamic acid synthesis
- Product Name:DL-Glutamic acid
- CAS Number:617-65-2
- Molecular formula:C5H9NO4
- Molecular Weight:147.13

110-89-4
3 suppliers
$15.96/100ML

617-65-2
273 suppliers
$6.00/25g
Yield:-
Reaction Conditions:
with benzotriazol-1-ol in dimethyl sulfoxide;N,N-dimethyl-formamide;
Steps:
3 Synthesis of Cyclic Peptide Cys-Lys-Asn-Tyr-Lys-[Lys-Thr-Glu(β Ala)]-Val (NH2-CKNYK-[KTE(β-A)]V-OH) (4)
Example 3
Synthesis of Cyclic Peptide Cys-Lys-Asn-Tyr-Lys-[Lys-Thr-Glu(β Ala)]-Val (NH2-CKNYK-[KTE(β-A)]V-OH) (4)
Fmoc-Val-Wang resin (1, 3.80 g, 1.25 mmol, 0.33 mmol/g) was swelled under dry nitrogen using anhydrous DMF for about 25 min.
The excess of the solvent was filtered off.
The swelling and filtration steps were repeated for 2 more times before the coupling reactions.
The Fmoc group was deprotected by using 20% piperidine in DMF two times (20% v/v, 2*125 mL, 25 min each) followed by extensive washing with DMF (6*50 mL) Fmoc-Glu(OPhipr)-OH (3 equiv, 1.83 g, 3.75 mmol) was coupled with the amino group of resin by using HBTU (1.42 g, 3.75 mmol, 3 equiv)/DIPEA (6 equiv, 1.31 mL) in DMF (20 mL) for 2 h.
A small amount of the resin was subjected to Kaiser test, which showed negative result indicating the coupling was completed.
The resin was washed extensively with DMF (6*50 mL).
The Fmoc group was deprotected by 20% piperidine in DMF as described above.
The subsequent coupling of amino acids, Fmoc-Thr(tBu)-OH, and Dde-Lys(Fmoc)-OH was carried out and finally Fmoc-βAla-OH was coupled to the side chain of K5 and the resin was washed DMF.
The PhiPr group of glutamic acid was removed using cocktail (TFA:ethanedithiol:DCM, 2:5:93, v/v/v, 125 mL) for 4*15 min.
The resin was washed with DMF and Fmoc group of the alanine was removed by using piperidine in DMF (20%, 2*25 min, 100 mL).
The resin was found to become aggregated due to the presence of positive and negative charged residues and hence was washed with DIPEA in DMF (3*3 min, 25 mL) that resulted in formation of DIPEA salts.
After washing the resin with DMF, the solvent was filtered.
The resin was allowed to agitate for 30 min in mixture of solvent, DMSO:NMP (1:4, 200 mL).
After resin beads looked uniform in the solvent, PyBOP/HOBT/DIPEA (3 equiv/3 equiv/6 equiv, 1.95 g/0.51 g/1.31 mL) were added in the solvent and allowed the resin beads to agitate for 2 h to afford 2.
After 2 h, small amount of beads was taken out and washed with DMF, DCM, and ethanol to perform Kaiser test, which showed negative result demonstrating that the cyclization was completed.
The formation of cyclic peptide was also confirmed by HR-MS (ESI-TOF): calcd. 692.3745; found 693.4215 [M+H]+).
References:
US2015/38671,2015,A1 Location in patent:Page/Page column
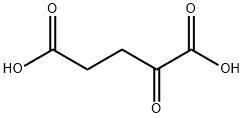
328-50-7
570 suppliers
$6.00/25g

617-65-2
273 suppliers
$6.00/25g
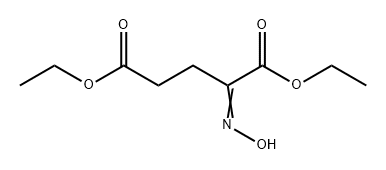
40418-75-5
0 suppliers
inquiry

617-65-2
273 suppliers
$6.00/25g