Diphenylacetonitrile synthesis
- Product Name:Diphenylacetonitrile
- CAS Number:86-29-3
- Molecular formula:C14H11N
- Molecular Weight:193.25
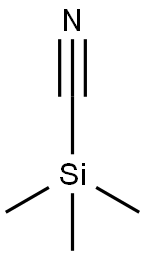
5798-79-8
49 suppliers
$55.00/50mg

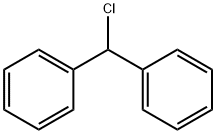
71-43-2
702 suppliers
$11.19/25ML

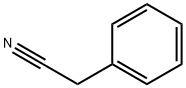
86-29-3
525 suppliers
$13.00/5g
Yield:86-29-3 585 g
Reaction Conditions:
Stage #1: bromo-phenyl-acetonitrile;benzene for 1 h;Reflux;
Stage #2: with aluminum (III) chloride at 20 - 40;
Steps:
2
After addition is complete, two liters of dry benzene was added and the mixture is healed under reflux for about one hour, until virtually ail the hydrogen bromide was escaped. The dropping funnel was instantly replaced by a solid rubber stopper.[00102| The reaction mixture was cooled to 20°C. Stirring is continued and 507 g (3.81 moles) of powdered anhydrous aluminum chloride was added in portions in the course of about one hour with the usual precautions. The temperaiure in this period was maintained at 20~25aC. When the addition of catalyst was complete, the temperature of the mixture was slowly raised. In about fifteen minutes., when the temperature has reached 35~40°C, vigorous evolution of hydrogen bromide commences, it was poured slowly and with stirring into a mixture of 1800 g. of ice and 760 ml, of 1 : 1 hydrochloric acid. The layers were separated. The aqueous portion is extracted twice with 800-mi. portions of benzene. The combined benzene extracts were washed successively with one liter of water, one liter of 5% sodium carbonate and one liter of water. The washings were discarded; the benzene solution was dried over 250g of anhydrous sodium sulfate. The benzene was distilled at atmospheric pressure and the residue was distilled under reduced pressure using a steam-heated condenser; bp 160-170X 5 nimHg. The crude product was reerystallized from methanol (0.5 niL/g); yield (in two crops) 585g (80% based on benzyl cyanide) ; mp 73-74X.
References:
WO2013/168000,2013,A1 Location in patent:Paragraph 00102
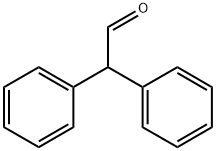
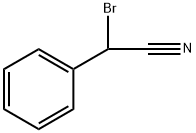
947-91-1
119 suppliers
$24.73/1gm:

86-29-3
525 suppliers
$13.00/5g