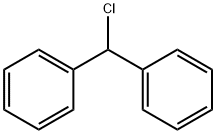
Chlorodiphenylmethane synthesis
- Product Name:Chlorodiphenylmethane
- CAS Number:90-99-3
- Molecular formula:C13H11Cl
- Molecular Weight:202.68

91-01-0
520 suppliers
$6.00/10g

90-99-3
303 suppliers
$9.00/5g
Yield:90-99-3 97%
Reaction Conditions:
with hydrogenchloride;tetrabutylammomium bromide in water;toluene at 40 - 45;
Steps:
General Method for the Preparation of 1-Benzhydrylpiperazine Derivatives (2a-c)
General procedure: NaBH4 (0.6mol) was added to a stirred solution of benzophenones (1.0 mol)in methanol (2 vol) portionwise at room temperature for 45 min. The mixture was stirred for 2-3 h at ambient temperature (completion of reaction was monitoredby TLC), and was diluted with water (750 ml), acidified with acetic acid to pH 4,and extracted with dichloromethane (2x400 ml). The organic layer was washed with water (200 ml) and dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to get pure benzhydrol derivatives as a white to off-white solid in 93-97% yield. To the benzhydrol derivatives (1.0 mol) in toluene (370 ml) was added concentrated HCl (35% in H2O, 370 ml) and tetrabutylammonium bromide(0.01 mol) at room temperature under stirring. Stirring was continued at 40-45 °C for 6-7 h. After completion of the reaction as indicated by TLC, the mixture was cooled to room temperature. The organic layer was separated and concentrated under vacuum to obtain crude benzhydryl chloride derivatives as a light brown liquid in 95-97% yield. To this benzhydryl chloride (0.96 mol) derivatives in toluene (380 ml) was added anhydrous piperazine (5.0 mol) at 60-70 °C for 45-60 min. The resulting mixture was heated under stirring at 90-100 °C for 8-10 h. The mixture was cooled after completion of the reaction. Water (380 ml) was added, and the organic layer was separated. The latter was washed with a 1:1 mixture of concentrated HCl/water(2x350 ml) and neutralized with 20% NaOH solution (750 ml). The water layer was re-extracted into toluene (2x300 ml), dried over anhydrous sodium sulfate, and concentrated under vacuum to result pure diphenylmethylpiperazine compounds (2a-c) as a white to off-white solid with yields up to 88%.
References:
Shivprakash;Reddy, G. Chandrasekara [Synthetic Communications,2014,vol. 44,# 5,p. 600 - 609] Location in patent:supporting information

91-01-0
520 suppliers
$6.00/10g

98-88-4
582 suppliers
$10.00/5g
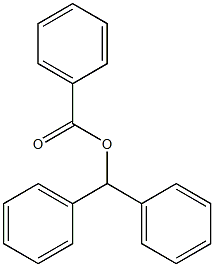
7515-28-8
0 suppliers
inquiry

90-99-3
303 suppliers
$9.00/5g
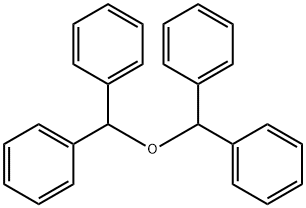
574-42-5
4 suppliers
$57.00/1ea

90-99-3
303 suppliers
$9.00/5g
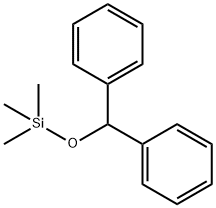
14629-59-5
2 suppliers
inquiry

90-99-3
303 suppliers
$9.00/5g
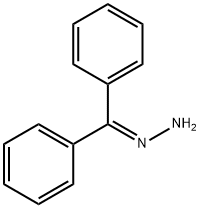
5350-57-2
425 suppliers
$5.00/10g

90-99-3
303 suppliers
$9.00/5g