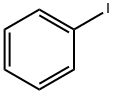
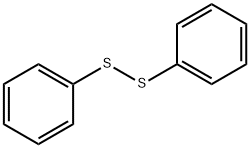
Diphenyl sulfide synthesis
- Product Name:Diphenyl sulfide
- CAS Number:139-66-2
- Molecular formula:C12H10S
- Molecular Weight:186.27
Yield:139-66-2 99%
Reaction Conditions:
with potassium tert-butylate;nickel 1,3-dibenzylimidazolidene in N,N-dimethyl-formamide at 110; for 16 h;Product distribution / selectivity;
Steps:
3
Different substrates were investigated(C)2-Ni catalyst Excellent activities for deactivated aryl iodides were observed. Quantitative yields were observed by using 1-1.5 mol% of Wi catalyst in DMP at 80oC for thiophenol (Table 4, entries 1-2) . For electron-rich aryl bromides, high activities were observed for (C)2-Ni. Low conversions and byproducts were observed for reactions of thiophenol with weaker bases (e.g., carbonate or phosphate) .
References:
WO2008/136770,2008,A1 Location in patent:Page/Page column 30-32