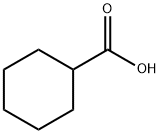
Cyclohexanecarboxylic acid synthesis
- Product Name:Cyclohexanecarboxylic acid
- CAS Number:98-89-5
- Molecular formula:C7H12O2
- Molecular Weight:128.17
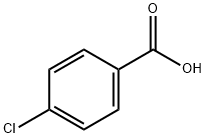
74-11-3
584 suppliers
$5.00/1g

98-89-5
385 suppliers
$13.00/25g
Yield:98-89-5 99 %Spectr.
Reaction Conditions:
with hydrogen in neat (no solvent) at 180; under 7500.75 Torr; for 24 h;Autoclave;Sealed tube;
Steps:
2.3. Typical procedures for catalytic hydrogenation
General procedure: All organic compounds (Tokyo Chemical Industry, Japan) were usedwithout further purification. Hydrogenation reactions were carried outin 10 mL autoclave with a glass tube inside equipped with magneticstirrer. In a typical experiment, 1 mmol of substrate and catalyst (1 mol%) were placed in the reactor and sealed. After being sealed, hydrogenwas introduced to reactor and inside of the reactor was purged formultiple times. The reactor was pressurized at 5-10 bar by hydrogen and heated to 100 °C-180 °C. After completion of reaction, the reactorwas cooled to room temperature and depressurized. The obtained re-action crude washed with acetone several times and resultant solutionwas filtered by using micro filters (Sartorius RC 0.20 μm). Then, thefiltrate solution was concentrated by using rotary evaporation.Conversion and yield of product were obtained by 1 H NMR analysisusing 1, 3, 5-trimethoxybenzene as an internal standard. 1 H NMRspectra were recorded using Brucker UltraShield 400 plus operating at400 MHz.
References:
Chaudhari, Chandan;Imatome, Hirotaka;Nishida, Yoshihide;Sato, Katsutoshi;Nagaoka, Katsutoshi [Catalysis Communications,2019,vol. 126,p. 55 - 60]
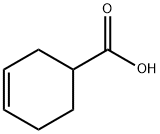
4771-80-6
280 suppliers
$5.00/10g

98-89-5
385 suppliers
$13.00/25g
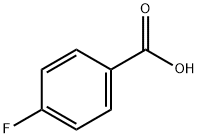
456-22-4
484 suppliers
$10.00/100 g

98-89-5
385 suppliers
$13.00/25g
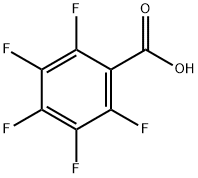
602-94-8
328 suppliers
$7.00/10g

98-89-5
385 suppliers
$13.00/25g
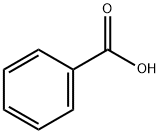
65-85-0
1372 suppliers
$10.00/25 g

98-89-5
385 suppliers
$13.00/25g