CINOXACIN synthesis
- Product Name:CINOXACIN
- CAS Number:28657-80-9
- Molecular formula:C12H10N2O5
- Molecular Weight:262.22

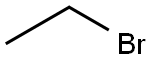
74-96-4
442 suppliers
$9.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
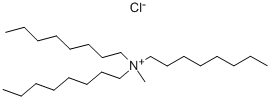
5137-55-3
433 suppliers
$5.00/10g

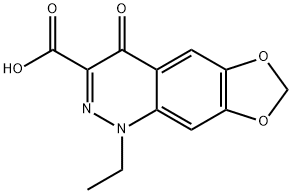
28657-80-9
114 suppliers
$56.00/100mg
Yield:-
Reaction Conditions:
with sodium hydroxide;potassium carbonate in water;toluene
Steps:
10 EXAMPLE 10
EXAMPLE 10 To a stirred suspension of 2.62 g 6,7-methylenedioxy-4(1H)-oxo-cinnolin-3-carboxylic acid ethylester, 2.8 g potassium carbonate, 1.4 g pulv. sodium hydroxide in 50 ml toluene, after addition of 0.4 g methyltrioctylammonium chloride, at 100° C. a solution of 1.52 ml ethylbromide in 5 ml toluene is added dropwise. After 2 hours and again after 3 hours, each time 0.76 ml ethyl bromide in 5 ml toluene are added dropwise. After a reaction period totalling 4 hours, the mixture is cooled to room temperature, 25 ml water are added, followed by 2 hours of stirring. The aqueous phase is then separated, filtered and acidified with hydrochloric acid. There precipitates a crude substance of 1-ethyl-6,7-methylenedioxy-4(1H)-oxo-cinnolin-3-carboxylic acid with a content of 80%, which is further purified through re-precipitation. Yield: 2.05 g.
References:
Veb Arzneimittelwerk Dresden US4495351, 1985, A