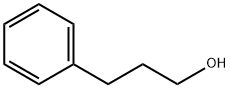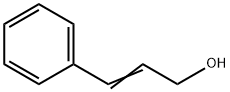
Cinnamyl alcohol synthesis
- Product Name:Cinnamyl alcohol
- CAS Number:104-54-1
- Molecular formula:C9H10O
- Molecular Weight:134.18

67-56-1
752 suppliers
$9.00/25ml
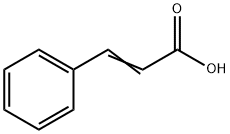
621-82-9
354 suppliers
$29.12/100G

104-54-1
629 suppliers
$13.00/25g
Yield:104-54-1 94%
Reaction Conditions:
with tert.-butylhydroperoxide in water at 110; for 4 h;Sealed tube;
Steps:
Activity test
General procedure: The typical experimental procedure for the decarboxylative coupling reaction was as follows: Cu-X catalyst (5mg), CA 1 (0.5mmol), TBHP (1.0mmol, 70% aqueous solution), and ethanol 2 (2.0mL) were placed in a sealed tube (10mL). The reaction was heated at 110°C for 4h. After the reaction finished, the catalyst was separated by centrifugation and filtration to obtain the liquid phase. The liquid products were analyzed by an Agilent 1260 Infinity Liquid Chromatogram. The pure product was obtained by flash column chromatography on silica gel using petroleum ether (60-90°C) and ethyl acetate as eluents. Compounds described in the literature were characterized by comparing their 1H and 13C NMR spectra and MS data with the reported data. The 1H NMR (500 and 400MHz) and 13C NMR (125 and 100MHz) were recorded with spectrometers at 20°C using CDCl3 as the solvent. Chemical shifts are given in parts per million relative to TMS as the internal standard at room temperature
References:
Chen, Shengchun;Shao, Zhen;Fang, Zhongxue;Chen, Qun;Tang, Ting;Fu, Wenqian;Zhang, Lei;Tang, Tiandi [Journal of Catalysis,2016,vol. 338,p. 38 - 46]
![Silane, (1,1-dimethylethyl)dimethyl[(3-phenyl-2-propenyl)oxy]-](/CAS/20180531/GIF/71700-50-0.gif)
71700-50-0
0 suppliers
inquiry

104-54-1
629 suppliers
$13.00/25g

621-82-9
354 suppliers
$29.12/100G

104-54-1
629 suppliers
$13.00/25g

104-55-2
654 suppliers
$6.00/25g

104-54-1
629 suppliers
$13.00/25g