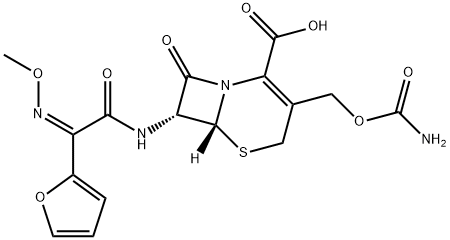
Cefuroxime synthesis
- Product Name:Cefuroxime
- CAS Number:55268-75-2
- Molecular formula:C16H16N4O8S
- Molecular Weight:424.39

Another simpler way of the synthesis of cefuroxime is by direct acylation of 7-amino- 3-aminocarbonyloxymethyl-3-cefem-4-carboxylic acid (32.1.2.22), which is isolated from the cultural fluid of Streptomyces lactamdurans, using syn-2-methoxyamino-2- (2-furyl)acetic acid chloride, which is synthesized by reacting the corresponding acid with phosphorous pentachloride.


1189-71-5
346 suppliers
$18.00/5g
![[6R-[6alpha,7beta(Z)]]-7-[2-furyl(methoxyimino)acetamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid](/CAS/GIF/56271-94-4.gif)
56271-94-4
70 suppliers
$139.90/2 mg

55268-75-2
251 suppliers
$49.00/25mg
Yield:55268-75-2 95%
Reaction Conditions:
Stage #1:isocyanate de chlorosulfonyle;(6R,7R)-7-[2-(2-furyl)-2-(methoxyimino)acetamido]-3-hydroxymethyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid in dimethylcarbonate at 0 - 5;
Stage #2: with hydrogenchloride;water in dimethylcarbonate at 10 - 15;Product distribution / selectivity;
Steps:
1
EXAMPLE 1; [00022] A 1:1 solution of chlorosulfonyl isocyanate (2.4 ml) in dimethylcarbonate was dropped into a suspension of (6R,7R)-7-[[2-furanyl(sin-methoxyimino)acetyl]amino]-3-hydroxymethyl-ceph-3-em-4-carboxylic acid (3.6 g) in dimethylcarbonate (35 ml) cooled to 0-4° C., under inert atmosphere, keeping the temperature below 5° C. [00023] When the addition of the reactive was completed, the mixture was kept at 0÷5° C. until the starting substrate was completely converted. [00024] 18% Hydrochloric acid (35 ml) was then added, keeping the heterogeneous mixture at a temperature ranging from 10 to 15° C. until the synthesis intermediate was completely hydrolysed. [00025] Cefuroxime acid was recovered by filtration in the form of a white crystalline powder (3.8 g) in a 95% yield, or Cefuroxime sodium salt was recovered by the method described in U.S. Pat. No. 4,775,750 in similar yields. [00026] The recovered product had high HPLC purity (>95%), and was characterized by 1H-NMR, 13C-NMR and mass spectroscopies, which gave the same data as those reported in literature for Cefuroxime.
References:
Antibioticos S.p.A. US6642378, 2003, B1 Location in patent:Page/Page column 2-3
![[6R-[6alpha,7beta(Z)]]-7-[2-furyl(methoxyimino)acetamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid](/CAS/GIF/56271-94-4.gif)
56271-94-4
70 suppliers
$139.90/2 mg
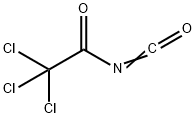
3019-71-4
157 suppliers
$26.30/1g

55268-75-2
251 suppliers
$49.00/25mg
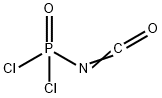
870-30-4
15 suppliers
$50.10/1g
![[6R-[6alpha,7beta(Z)]]-7-[2-furyl(methoxyimino)acetamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid](/CAS/GIF/56271-94-4.gif)
56271-94-4
70 suppliers
$139.90/2 mg

55268-75-2
251 suppliers
$49.00/25mg

957-68-6
438 suppliers
$14.00/5g

55268-75-2
251 suppliers
$49.00/25mg
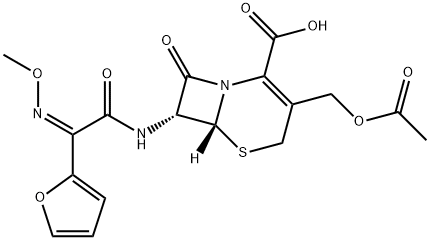
39685-31-9
63 suppliers
inquiry

55268-75-2
251 suppliers
$49.00/25mg