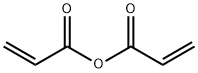
Acrylic anhydride synthesis
- Product Name:Acrylic anhydride
- CAS Number:2051-76-5
- Molecular formula:C6H6O3
- Molecular Weight:126.11
In a 3-liter, four-necked glass reactor provided with a thermometer and a stirrer were placed, in a dry air atmosphere, 1,240 g of methylene chloride, 1.08 g (0.5% by mass) of Sumilizer GM, 0.65 g (0.3% by mass) of Sumilizer TP-D, 0.65 g (0.3% by mass) of Sumilizer WX-R, 216 g (3.0 mol) of acrylic acid and 264 g (1.5 mol) of benzenesulfonyl chloride. The mixture was cooled to 5°C. Then, 304 g (3.0 mol, 1 equivalent relative to the acids generated from benzenesulfonyl chloride) of triethylamine was dropwise added in 2 hours with the temperature of the reaction mixture being controlled at 30°C or lower. After the completion of the dropwise addition, stirring was made for 1 hour with the same temperature being kept. The reaction mixture was analyzed by GC, which indicated that the conversion of acrylic acid was 99%. After the completion of the reaction, 375 g of water was added to the reaction mixture to wash the reaction mixture. The reaction mixture was further washed twice each time with 563 g of water, after which distillation was conducted to remove methylene chloride. The yield of the acrylic acid anhydride obtained was 172 g and 91% and its purity by GC analysis was 99%, and no high-molecular by-product was detected.

79-10-7
699 suppliers
$20.50/500ml

2051-76-5
165 suppliers
$20.00/1G
Yield:2051-76-5 95%
Reaction Conditions:
with trifluorormethanesulfonic acid;acetic anhydride at 100;
Steps:
3 Example 3
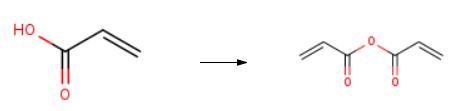
Acrylic anhydride CH2═CH-C(═O)-O-(O═)C═CH═CH was prepared according to the process of the invention by reaction between acrylic acid and acetic anhydride. The sequence of appliances is represented in FIG. 3. (0114) In the reaction region, the reaction temperature was set at 100° C. and the pressure was set at atmospheric pressure. (0115) In the distillation (extraction) region, the pressure was set at 20 mbar absolute and a temperature gradient (between 90° C. at the column bottom and 25° C. at the column top) was observed. (0116) As indicated in FIG. 3, the reaction region was fed with the stream 1, with an overall flow rate of 2 kg/h and composed of the recycling stream 5 and of a continuous feed of a fresh mixture of acetic anhydride, acrylic acid and triflic acid. (0117) The adjustments of the stream 1, composed of the stream of fresh mixture and of the stream 5, were such that, in the reaction region, there was continuously: (0118) acetic anhydride: incoming flow rate of 795 g/h (7.794 mol/h), (0119) acrylic acid: incoming flow rate of 1004 g/h (13.95 mol/h), and (0120) triflic acid at 50 ppm by weight, with respect to the combination {acetic anhydride+acrylic acid}. (0121) At the outlet of this reaction region, the stream 2 essentially conveyed acrylic anhydride, acetic acid, triflic acid and mixed acrylic/acetic anhydride towards the distillation column. (0122) Under the conditions described above, the acrylic anhydride, with a purity of greater than 95%, was recovered in the stream 3 with a flow rate of approximately 840 g/h (6.66 mol/h). At the column top, the stream 4 (950 g/h, 14.8 mol/h) was very predominantly (>95% by weight) composed of acetic acid. The recycling stream 5 essentially transported triflic acid and mixed acrylic/acetic anhydride in order to reinject them into the reaction region.
References:
US9266811,2016,B2 Location in patent:Paragraph 0113-0122

79-10-7
699 suppliers
$20.50/500ml
814-68-6
383 suppliers
$21.21/5gm:

2051-76-5
165 suppliers
$20.00/1G
10192-85-5
102 suppliers
$7.00/5g

2051-76-5
165 suppliers
$20.00/1G
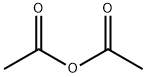
108-24-7
5 suppliers
$14.00/250ML

79-10-7
699 suppliers
$20.50/500ml

2051-76-5
165 suppliers
$20.00/1G
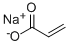
7446-81-3
191 suppliers
$5.00/1g

814-68-6
383 suppliers
$21.21/5gm:

2051-76-5
165 suppliers
$20.00/1G