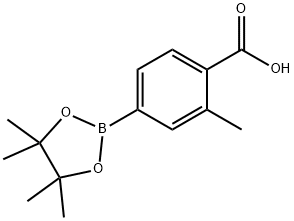
2-METHYL-4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)BENZOIC ACID synthesis
- Product Name:2-METHYL-4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)BENZOIC ACID
- CAS Number:890839-22-2
- Molecular formula:C14H19BO4
- Molecular Weight:262.11
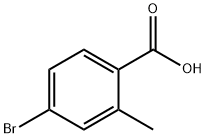
68837-59-2
339 suppliers
$5.00/1g
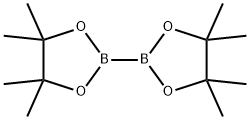
73183-34-3
570 suppliers
$6.00/5g

890839-22-2
30 suppliers
$115.00/50mg
Yield:890839-22-2 73%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 90;Inert atmosphere;
Steps:
1-methyl-4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-benzoic acid (102)
(0405) Potassium acetate (1473 mg, 15.00 mmol), bis-(pinacolato)-diboron (1523 mg, 6.00 mmol) and bis(diphenylphosphine) ferrocene dichloropalladium (II) complex with dichloromethane (183 mg, 0.25 mmol) was added to an anhydrous solution of 4-bromo-2-methylbenzoic acid (1075 mg, 5.00 mmol) in dioxane (180 mL) under anhydrous conditions in an atmosphere of nitrogen. The mixture was stirred at 90 °C overnight. The reaction was then quenched with water and extracted with ethyl acetate. The combined organic layers were dried over magnesium sulfate, filtered and concentrated. The residue was purified by flash column chromatography on silica gel (eluent: (0406) dichloromethane/ethyl acetate, 10-100%) to give the title compound as a white solid (970 mg, 73%). ESI-MS m/z: 285.1284 [M+H]+; 1H NMR (400 MHz, DMSO-<) δ 7.79 (d, J= 7.6 Hz, (0407) 1H), 7.59 - 7.54 (m, 2H), 2.52 (s, 3H), 1.30 (s, 12H). 13C NMR (101 MHz, DMSO) δ 168.70, 162.31, 137.91, 137.42, 133.30, 131.70, 129.35, 83.94, 24.67, 20.94.
References:
WO2019/89648,2019,A1 Location in patent:Page/Page column 55