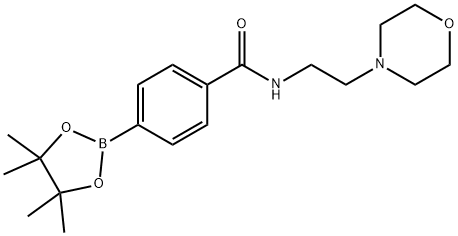
4-(2-[4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-PHENOXY]-ETHYL)-MORPHOLINE synthesis
- Product Name:4-(2-[4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-PHENOXY]-ETHYL)-MORPHOLINE
- CAS Number:787591-39-3
- Molecular formula:C19H29BN2O4
- Molecular Weight:360.26
2038-03-1
206 suppliers
$27.00/25g
180516-87-4
231 suppliers
$8.00/1g
![4-(2-[4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-PHENOXY]-ETHYL)-MORPHOLINE](/CAS/GIF/787591-39-3.gif)
787591-39-3
32 suppliers
$45.00/250mg
Yield:787591-39-3 53%
Reaction Conditions:
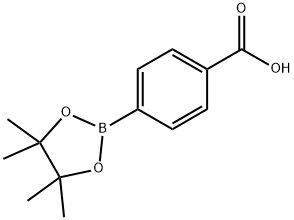
Stage #1: 4-carboxyphenylboronic acid pinacol esterwith triethylamine;isobutyl chloroformate in dichloromethane at -15; for 0.0833333 h;
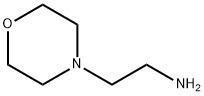
Stage #2: 4-(2-AMINOETHYL)MORPHOLINE in dichloromethane at -15 - 20; for 0.166667 h;
Steps:
90.1 Example 90; 4-(8-Methylamino-imidazo[1,2-a]pyrazin-3-yl)-N-(2-morpholin-4-yl-ethyl)-benzamide
In a 2 dram vial under nitrogen, 500 mg (2.0 mmol) of E was added and dissolved in dichloromethane (DCM) (5 mL). 606 mg (6.0 mmol) of triethyl amine (TEA) was then added via syringe and the stirring mixture cooled to -15° C. 272 mg (2.0 mmol) of isobutyl chloroformate (IBC) was then added dropwise and the reaction mixture stirred for 5 minutes. After 5 minutes the formation of a white precipitate was observed, at which point the amine F was added and the solution allowed to warm to room temperature. Upon warming, the reaction was stirred for an additional 10 minutes. The reaction mixture was then diluted with dichloromethane (100 mL) and extracted with a saturated solution of sodium bicarbonate (2*25 mL). The organic layer was then dried over Na2SO4, filtered, and concentrated under vacuum. The colorless oil was then triturated using hexanes (50 mL) to provide G as a white solid. Yield: 53%. 1H NMR (300 MHz, CDCl3) δ 7.87 (d, J=8.2, 2H), 7.75 (d, J=8.2, 2H), 6.75 (br. s, 1H), 3.72 (t, J=4.6, 4H), 3.15 (q, J=5.7, 2H) 2.61 (t, J=6.0, 2H) 2.51 (t, J=4.5, 4H), 1.35 (s, 12H).
References:
US2004/220189,2004,A1 Location in patent:Page 45-46