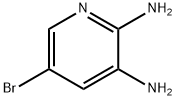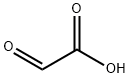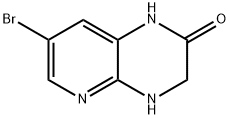
7-BROMO-3,4-DIHYDROPYRIDO[2,3-B]PYRAZIN-2(1H)-ONE synthesis
- Product Name:7-BROMO-3,4-DIHYDROPYRIDO[2,3-B]PYRAZIN-2(1H)-ONE
- CAS Number:709652-84-6
- Molecular formula:C7H6BrN3O
- Molecular Weight:228.05
Yield:709652-84-6 52%
Reaction Conditions:
Stage #1: 5-bromo-pyridine-2,3-diamine;Glyoxilic acid in methanol; for 62 h;
Stage #2: with sodium tris(acetoxy)borohydride in 1,2-dimethoxyethane at 60; for 88 h;
Steps:
161.a Example 161; Preparation of (E)-N-METHYL-N-(L-METHYL-LH-INDOL-2-YLMETHYL .-3-(2-OXO-1 2. 3 4- TETRAHYDRO-PVRIDO- [2, 3-B] PYRAZIN-7-YL)-ACRYLAMIDE; : a) 7-bromo-3, 4-dihydro-lH-pyrido [2, 3-B] pyrazin-2-one
A mixture OF 5-BROMO-2, 3-diaminopyridine (11.64 g, 61.9 mmol) and glyoxylic acid monohydrate (22.80 g, 247.7 mmol) in MEOH (200 mL) was stirred for 62 hours. The precipitate was filtered, washed with MEOH and dried at 110 °C to give 12.60 g (90%) of a regioisomeric mixture of the condensation products. The mixture (4.52 g, 20 mmol) was suspended in DME (300 mL) and, after addition OF NABH (OAC) 3 (11.87 g, 56 mmol), it was stirred for 88 hours at 60 °C. Upon cooling, EtOAc (500 mL) and water (300 mL) was added and the pH was adjusted to 8.0 with 2N NaOH. The aqueous phase was separated and extracted with EtOAc (2X200 mL). The combined organic phases were washed with water and brine, dried and evaporated. The residue was stirred with CH2C12 (50 mL) for 24 hr then filtered. The solid cake was stirred with EtOAc (100 mL) at 75 °C for 14 hours, filtered and dried to afford the title compound (2.35 g, 52%). 1H. NMR (300 MHz, DMSO- d6) 8 10.47 (s, br, 1H), 7.65 (d, J= 2.2Hz, 1H), 7.01 (t, J= 2.2Hz, 1H), 6.99 (s, br, 1H), 3.93 (d, J= 1. 5HZ, 2H). MS (ESI) m/e : 227.9764 (M+H) +.
References:
WO2004/52890,2004,A1 Location in patent:Page 189
67443-38-3
368 suppliers
$5.00/1g
![7-BROMO-3,4-DIHYDROPYRIDO[2,3-B]PYRAZIN-2(1H)-ONE](/CAS/GIF/709652-84-6.gif)
709652-84-6
18 suppliers
inquiry