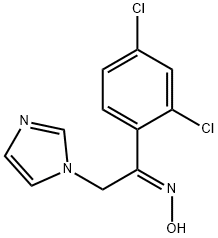
(Z)-2'-(1H-Imidazole-1-yl)-2,4-dichloroacetophenone oxime synthesis
- Product Name:(Z)-2'-(1H-Imidazole-1-yl)-2,4-dichloroacetophenone oxime
- CAS Number:64211-06-9
- Molecular formula:C11H9Cl2N3O
- Molecular Weight:270.11

288-32-4
1008 suppliers
$5.00/25g

4252-78-2
363 suppliers
$9.00/25g

64211-06-9
57 suppliers
$67.50/250mg
Yield:-
Reaction Conditions:
Stage #1: 1H-imidazole;2,2',4'-trichloroacetophenone in methanol; for 3.83333 h;Reflux;
Stage #2: with ammonium hydroxide;dihydrogen peroxide in methanol at 25; for 5 h;Reagent/catalyst;
Steps:
1-6 Example 5
The first step is to add 1mmol 2-chloro-1-(2,4-dichlorophenyl)ethanone, 1mmol imidazole and 38mL anhydrous methanol to a three-necked flask. After stirring for 20min at room temperature, the reaction is heated and refluxed for 3.5h, and the reaction is complete. Afterwards, add 2mL hydrogen peroxide, 5mL ammonia monohydrate and the modified catalyst of Example 2, the reaction temperature is 25, the rotating speed is 120r/min, and the reaction is stirred for 5h. After the reaction is over, double volume of deionized water is added to terminate the reaction and filtered. The filtrate was extracted with ethyl acetate, and the ethyl acetate was removed by rotary evaporation to obtain a crude product. The crude product was recrystallized from ethylene glycol monomethyl ether and dried under vacuum at 75°C for 3 hours to obtain a purified product. After the purified product was subjected to light treatment, quality control was performed. Obtain (Z)-2'-(1H-imidazol-1-yl)-2,4-dichloroacetophenone oxime with higher purity
References:
CN113387891,2021,A Location in patent:Paragraph 0047-0049; 0055-0057; 0059-0060; 0063-0065; ...

288-32-4
1008 suppliers
$5.00/25g
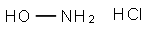
5470-11-1
772 suppliers
$10.00/5G

46503-52-0
103 suppliers
inquiry

4252-78-2
363 suppliers
$9.00/25g

64211-06-9
57 suppliers
$67.50/250mg

4252-78-2
363 suppliers
$9.00/25g

64211-06-9
57 suppliers
$67.50/250mg