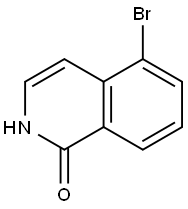
5-BROMOISOQUINOLIN-1(2H)-ONE synthesis
- Product Name:5-BROMOISOQUINOLIN-1(2H)-ONE
- CAS Number:190777-77-6
- Molecular formula:C9H6BrNO
- Molecular Weight:224.05
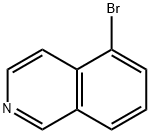
34784-04-8
353 suppliers
$7.00/1g

190777-77-6
118 suppliers
$37.00/250mg
Yield:190777-77-6 43%
Reaction Conditions:
Stage #1:5-bromoisoquinoline with 3-chloro-benzenecarboperoxoic acid in dichloromethane at 20; for 0.5 h;
Stage #2: with acetic anhydride for 1 h;Reflux;
Steps:
30
Commercially available 5-bromoisoquinoline (4.85 g, 23.3 mmol) was dissolved in dichloromethane (78 mL), and m-CPBA (9.28 g, 35.0 mmol) was added. The mixture was stirred at room temperature for 0.5 hours. The reaction mixture was diluted by adding chloroform, and washed with a saturated sodium bicarbonate aqueous solution and saturated brine. The organic layer was dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The obtained residue was dissolved in acetic anhydride (78.0 mL), and stirred for 1 hour under reflux. A 2.0 mol/L sodium hydroxide aqueous solution (156 mL) was added to the residue obtained by concentrating the reaction mixture under reduced pressure, and the mixture was stirred for 2 hours under reflux. The reaction mixture was cooled to room temperature, and neutralized with a 2.0 mol/L hydrochloric acid aqueous solution. The precipitated crystals were collected by filtration, and dried under reduced pressure to give 5-bromoisoquinolin-1(2H)-one (2.28 g, 10.1 mmol, 43%).
References:
Kyowa Hakko Kirin Co., Ltd.;OTSUBO, Nobumasa;OKAZAKI, Shuko;TSUKUMO, Yukihito;IIDA, Kyoichiro;NAKOJI, Masayoshi EP2708540, 2014, A1 Location in patent:Paragraph 0122
1109230-25-2
83 suppliers
$38.00/100mg

190777-77-6
118 suppliers
$37.00/250mg
245677-36-5
27 suppliers
inquiry

190777-77-6
118 suppliers
$37.00/250mg
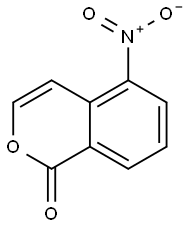
77747-69-4
18 suppliers
inquiry

190777-77-6
118 suppliers
$37.00/250mg

15115-60-3
331 suppliers
$5.00/1g

190777-77-6
118 suppliers
$37.00/250mg