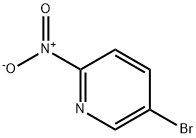
5-Bromo-2-nitropyridine synthesis
- Product Name:5-Bromo-2-nitropyridine
- CAS Number:39856-50-3
- Molecular formula:C5H3BrN2O2
- Molecular Weight:202.99
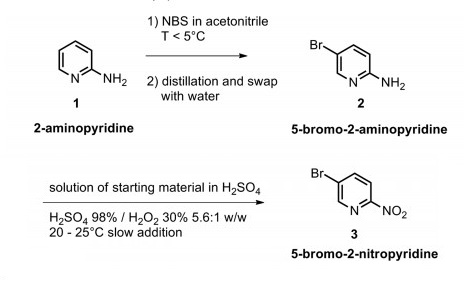
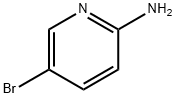
1072-97-5
688 suppliers
$9.00/1g

39856-50-3
535 suppliers
$6.00/5g
Yield:39856-50-3 92.8%
Reaction Conditions:
with dihydrogen peroxide in water;acetone at 10 - 40; for 6 h;Reagent/catalyst;Solvent;Temperature;
Steps:
2-3; 4.1-4.4; 5.1-5.4
Add 2-amino-5-bromopyridine (173g, 1.0mol), catalyst S/ZW (26.0g, 15.0wt%), 1.5L acetone/water mixture (volume ratio acetone/water) into a 5L three-neck glass bottle =7/1) Stir uniformly with a mechanical stirrer at 20-30°C, then control the temperature of the reaction system to 10-15°C, and use a constant pressure dropping funnel to drop 30wt% H2O2 (3.5mol, 3.0eq);2) After the addition of H2O2, heat the reaction at 10-15°C for 1-2h, then heat it up to 30°C at a heating rate of 2°C/min for 2h, and finally heat it to 40°C at a heating rate of 1°C/min until The concentration of the substrate 2-amino-5-bromopyridine is no longer decreased by HPLC;(Reaction at 40°C for 4 hours, HPLC detection of material area percentage: substrate 0.18%, product 99.4%, dibromide by-product 0.16%, and other unknown impurities sum 0.26%;The result of the small-scale reaction is not much different, indicating that the oxidation reaction of the present invention can be scaled up according to the existing process);3) Cool down to room temperature, filter to remove the catalyst, add sodium dithionite to the filtrate and stir for 1-2h;4) Use starch potassium iodide test paper to test that there is no excess oxidant in the system, then heat up to 45-55°C and stir for 10-30 minutes, then add alkaline aqueous solution to adjust the pH of the system to 8.0, and finally add dropwise anti-solvent 2.0L (volume ratio, purified water/iso) Propanol = 4/1) Crystallize, cool to room temperature and filter,After drying, 188.4 g of 2-nitro-5-bromopyridine was obtained (the yield was 92.8%),The purity detected by HPLC was 99.82%, and the largest single impurity was 0.06% by-product of dibromide.
References:
CN112645870,2021,A Location in patent:Paragraph 0030-0069
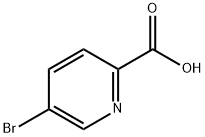
30766-11-1
394 suppliers
$3.00/1g

39856-50-3
535 suppliers
$6.00/5g
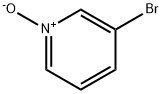
2402-97-3
122 suppliers
$30.00/1g

39856-50-3
535 suppliers
$6.00/5g
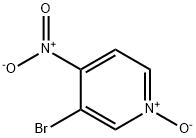
1678-49-5
135 suppliers
$31.00/1g
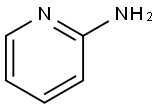
504-29-0
548 suppliers
$5.00/5G

39856-50-3
535 suppliers
$6.00/5g

504-29-0
548 suppliers
$5.00/5G
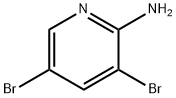
35486-42-1
368 suppliers
$10.00/5g

39856-50-3
535 suppliers
$6.00/5g