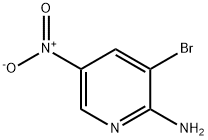
2-Amino-3-bromo-5-nitropyridine synthesis
- Product Name:2-Amino-3-bromo-5-nitropyridine
- CAS Number:15862-31-4
- Molecular formula:C5H4BrN3O2
- Molecular Weight:218.01
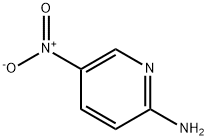
4214-76-0
519 suppliers
$6.00/10g

15862-31-4
247 suppliers
$5.00/1g
Yield:15862-31-4 32%
Reaction Conditions:
Stage #1: 5-nitro-pyridin-2-ylaminewith bromine;acetic acid at 20;
Stage #2: with sodium hydrogencarbonate in water;ethyl acetate; pH=8 - 9;Saturated solution;
Steps:
5.a
Step a: 3-Bromo-5-nitropyridin-2-amine To a solution of 5-nitro-pyridin-2-ylamine (30 g, 0.22 mol) in acetic acid (200 mL) at 10° C. was added Br2 (38 g, 0.24 mol) dropwise. After addition, the mixture was stirred at 20° C. for 30 min. The solid was filtered and then dissolved in ethyl acetate (200 mL). The mixture was basified to pH 8-9 with saturated aqueous NaHCO3. The organic layer was separated, and the aqueous layer was extracted with ethyl acetate (100 mL*3). The combined organic layers were washed with water, brine, dried over Na2SO4 and concentrated under vacuum to afford 3-bromo-5-nitropyridin-2-amine (14.8 g, 32%). 1H-NMR (CDCl3, 400 MHz) δ 8.94 (d, J=2.4 Hz, 1H), 8.50 (d, J=2.4 Hz, 1H), 5.67 (brs, 2H).
References:
US2009/253736,2009,A1 Location in patent:Page/Page column 25
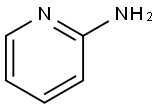
504-29-0
549 suppliers
$5.00/5G

15862-31-4
247 suppliers
$5.00/1g
