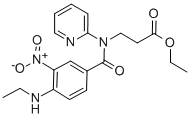
ETHYL N-[4-(METHYLAMINO)-3-NITROBENZOYL]-N-PYRIDIN-2-YL-SS-ALANINATE synthesis
- Product Name:ETHYL N-[4-(METHYLAMINO)-3-NITROBENZOYL]-N-PYRIDIN-2-YL-SS-ALANINATE
- CAS Number:429659-01-8
- Molecular formula:C18H20N4O5
- Molecular Weight:372.38
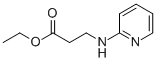
103041-38-9
380 suppliers
$6.00/1g
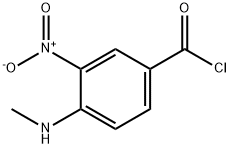
82357-48-0
39 suppliers
inquiry
![ETHYL N-[4-(METHYLAMINO)-3-NITROBENZOYL]-N-PYRIDIN-2-YL-SS-ALANINATE](/CAS/GIF/429659-01-8.gif)
429659-01-8
185 suppliers
$12.00/5g
Yield:429659-01-8 180 g
Reaction Conditions:
Stage #1: 4-methylamino-3-nitro-benzoic acid chloridewith triethylamine in dichloromethane at 0 - 10;
Stage #2: 2-chloromethyl-1-methyl-1H-benzimidazole-5 ethyl acetate in dichloromethane at 0 - 30;
Steps:
c c) Ethyl3- { F U -(methylamino)-2-nitrophen-4-yl} carbonyll (pyridyn-2- yl)aminolpropanoate (IX):
100 g (0.SOmol) of 4-(methylamino)-3-nitrobenzoic acid as obtained in step (a) was suspended in 800 ml dichioromethane. To the obtained suspension was added of 200 ml of thionyl chloride diluted with 200 ml dichioromethane. Finally to the reaction mixture was added 2 ml of N,N-dimethylformamide. The mixture was stirred at reflux temperature for 4-5 hr. completion of reaction was monitored by TLC. Excess thionyl chloride was removed by vacuum distillation. The residue was dissolved in 100 ml of dichioromethane which was distilled under vacuum. Finally to the obtained residue was charged 700 ml of dichioromethane and solution was cooled to 0-5°C. To the cooled solution was added mixture of2l3 ml triethylamine and 100 ml ?0dichloromethane at 0-10°C temperature and stirred fQr 10-15 mm. Finally to thereaction mixture was added 93 gm (0.48 mol) Ethyl 3-(2-pyridylamino)propanoate dissolved in 200 ml dichloromethane at 0-10°C. temperature and stirred for 10-15 mm. Reaction mixture was then warmed to 25-30°C and stirred for 4-5 hr and ‘completion of reaction was monitored by TLC. Upon completion of reaction, reaction mixture was quenched with 1.0 Lit purified water at 0-10°C and stirred for 30 mm. Layers were then allowed to separate for 30 mm. Organic layer was separated and was washed with purified water 1.0 Lit at 0-10°C. Finally organic layer was washed with 2% HC1 solution 1.0 Lit X 3 times. The obtained organic layer was then washed with 10% sodium bicarbonate solution 1.0 lit X 2 times. Dichloromethane layer was then distilled under vacuum to obtain crude Ethyl3 - { [{ 1-(methylamino)-2-nitrophen-4-yl } carbonyl] (pyridyn-2-yl)aminol propanoate. Yield: 180 g (94.82%); Purity by HPLC: 97.6 %.
References:
WO2015/128875,2015,A2 Location in patent:Page/Page column 41; 42
![3-[(4-chloro-3-nitrobenzoyl)pyridin-2-yl-amino]propionic acid ethyl ester](/CAS/20200119/GIF/1620205-04-0.gif)
1620205-04-0
5 suppliers
inquiry
74-89-5
7 suppliers
$13.44/25ML
![ETHYL N-[4-(METHYLAMINO)-3-NITROBENZOYL]-N-PYRIDIN-2-YL-SS-ALANINATE](/CAS/GIF/429659-01-8.gif)
429659-01-8
185 suppliers
$12.00/5g

103041-38-9
380 suppliers
$6.00/1g
![ETHYL N-[4-(METHYLAMINO)-3-NITROBENZOYL]-N-PYRIDIN-2-YL-SS-ALANINATE](/CAS/GIF/429659-01-8.gif)
429659-01-8
185 suppliers
$12.00/5g

103041-38-9
380 suppliers
$6.00/1g
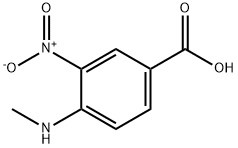
41263-74-5
323 suppliers
$9.00/5g
![ETHYL N-[4-(METHYLAMINO)-3-NITROBENZOYL]-N-PYRIDIN-2-YL-SS-ALANINATE](/CAS/GIF/429659-01-8.gif)
429659-01-8
185 suppliers
$12.00/5g

41263-74-5
323 suppliers
$9.00/5g
![ETHYL N-[4-(METHYLAMINO)-3-NITROBENZOYL]-N-PYRIDIN-2-YL-SS-ALANINATE](/CAS/GIF/429659-01-8.gif)
429659-01-8
185 suppliers
$12.00/5g