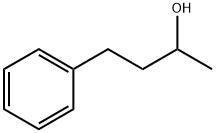
4-Phenyl-2-butanol synthesis
- Product Name:4-Phenyl-2-butanol
- CAS Number:2344-70-9
- Molecular formula:C10H14O
- Molecular Weight:150.22

1896-62-4
225 suppliers
$11.00/100g

2344-70-9
152 suppliers
$12.00/1g
Yield:2344-70-9 99%
Reaction Conditions:
(HN(iPr2PC2H4)2)IrH3 in isopropyl alcohol at 25; for 1 - 2 h;Conversion of starting material;
Steps:
5.1 Example 5.1 Transfer hydrogenation of benzylidene acetone using IrH3 ((IPR2PC2H4) 2NH) as catalyst
A weighed amount of IRH3 ((IPR2PC2H4) 2NH) is added to a solution of benzylidene acetone in 2-propanol and the mixture stirred at the required temperature. The reaction progress is monitored using NMR. After attainment of an equilibrium conversion, the solvent is removed by evaporation under reduced pressure. A typical example is illustrated below: IrH3 ((LPR2PC2H4) 2NH) (5 mg) is added to a solution of benzylidene acetone (2.0 g) in 2-propanol (10 g) and the reaction mixture stirred for 1 hour at room temperature. The solvent was evaporated under reduced pressure, resulting in > 99% of the saturated alcohol. The results are presented in Table 6.
References:
ABDUR-RASHID, Kamaluddin WO2004/96735, 2004, A2 Location in patent:Page 21-22
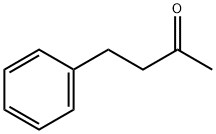
2550-26-7
348 suppliers
$9.00/100g

2344-70-9
152 suppliers
$12.00/1g

1896-62-4
225 suppliers
$11.00/100g

2550-26-7
348 suppliers
$9.00/100g

2344-70-9
152 suppliers
$12.00/1g
180295-18-5
0 suppliers
inquiry

2344-70-9
152 suppliers
$12.00/1g
36004-04-3
4 suppliers
inquiry

2344-70-9
152 suppliers
$12.00/1g