
4-PHENOXYPHENYLBORONIC ACID synthesis
- Product Name:4-PHENOXYPHENYLBORONIC ACID
- CAS Number:51067-38-0
- Molecular formula:C12H11BO3
- Molecular Weight:214.02
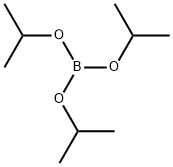
5419-55-6
377 suppliers
$12.00/5g

2050-47-7
263 suppliers
$6.00/5g

7732-18-5
471 suppliers
$12.69/100ml

51067-38-0
344 suppliers
$9.00/1g
Yield:51067-38-0 91%
Reaction Conditions:
Stage #1:Triisopropyl borate;4,4'-dibromodiphenyl ether with n-butyllithium in tetrahydrofuran;hexane at -70 - 20;Inert atmosphere;
Stage #2:water with hydrogenchloride in tetrahydrofuran;hexane at 0;Inert atmosphere;Solvent;
Steps:
1-5
Under nitrogen protection, mix the above 4,4'-dibromodiphenyl ether (16.4g, 0.05mol), tetrahydrofuran (160mL) and triisopropyl borate (10.3g, 0.055mol), and cool to -70°C to- At 60°C, start to add 44 mL of 2.5M n-butyl lithium hexane solution dropwise. After the dripping is completed, keep it warm and stir for 1 hour. Then it was naturally raised to room temperature and reacted overnight. After cooling, 1M hydrochloric acid was added for quenching, and the quenching process did not exceed 0°C. Extract twice with tetrahydrofuran (60mL), combine the oil layers, evaporate to dryness under reduced pressure, add ethyl acetate to re-dissolve, filter, and evaporate to dryness again. After beating with n-heptane/methyl tert-butyl ether (10/1), the off-white is obtained Solid 9.7 g, yield 91%, HPLC: 99.9%, HNMR structure is consistent.
References:
Cangzhou Purui Eastern Countries Technology Co., Ltd.;Zhang Jin;Leng Yanguo;Chang Zhiliang;Feng Xuemin;Meng Qingbin CN111072697, 2020, A Location in patent:Page/Page column 4-6
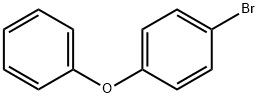
101-55-3
256 suppliers
$8.00/5g

121-43-7
356 suppliers
$13.00/25mL

7732-18-5
471 suppliers
$12.69/100ml

51067-38-0
344 suppliers
$9.00/1g

101-55-3
256 suppliers
$8.00/5g

51067-38-0
344 suppliers
$9.00/1g
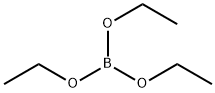
150-46-9
197 suppliers
$12.00/25mL
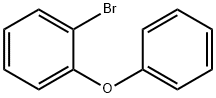
7025-06-1
65 suppliers
$47.00/1g

51067-38-0
344 suppliers
$9.00/1g