
4-Ethyl-2-methoxyphenol synthesis
- Product Name:4-Ethyl-2-methoxyphenol
- CAS Number:2785-89-9
- Molecular formula:C9H12O2
- Molecular Weight:152.19
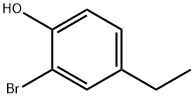
64080-15-5
58 suppliers
$60.00/100mg
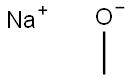
124-41-4
690 suppliers
$12.00/25g

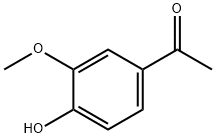
2785-89-9
396 suppliers
$5.00/1g
Yield:2785-89-9 98%
Reaction Conditions:
with methanol;Methyl formate;copper(l) chloride at 115; for 2 h;Autoclave;Green chemistry;
Steps:
Typical procedure for synthesis of 1 (Table 1)
General procedure: A Teflon-lined autoclave (25 mL) was charged with MeONa (1.08 g, 20.0 mmol), MeOH (10 mL), CuCl (40 mg, 0.40 mmol), HCOOMe (0.25 mL, 0.97 g/mL, 4.0 mmol), and monohaloarene (10.0 mmol) then heated to 115 °C, with stirring, for 2 h. After completion of the reaction, the reactor was cooled to room temperature. The mixture was stirred for 0.5 h in the open, then concentrated to recover pure MeOH. Diethyl ether (15 mL) and dilute hydrochloric acid (1.6 M, 15 mL) were added to the residue. The mixture separated into two layers, and the aqueous phase was extracted with diethyl ether (15 mL x 3). The combined organic layers were dried over anhydrous Na2SO4 and concentrated to give a residue which was purified by column chromatography on silica gel (mobile phase: petroleum ether-ethyl acetate 15:1) to furnish 1 (conversion and selectivity were determined by GC-MS analysis). The purity of the recovered MeOH was measured as more than 99 % by GC, and the water content of the recovered MeOH was measured as less than 0.12 % by use of the Karl Fischer method.
References:
Guo, Ying;Ji, Si-Zhe;Chen, Cheng;Liu, Hong-Wei;Zhao, Jian-Hong;Zheng, Yu-Lin;Ji, Ya-Fei [Research on Chemical Intermediates,2015,vol. 41,# 11,p. 8651 - 8664]
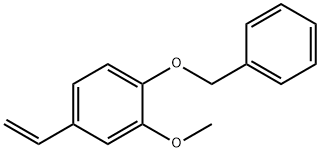
498-02-2
460 suppliers
$5.00/1g

2785-89-9
396 suppliers
$5.00/1g
55708-65-1
32 suppliers
$83.50/1g

2785-89-9
396 suppliers
$5.00/1g
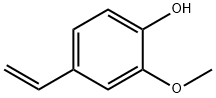
7786-61-0
302 suppliers
$13.00/1g

2785-89-9
396 suppliers
$5.00/1g

498-02-2
460 suppliers
$5.00/1g

7786-61-0
302 suppliers
$13.00/1g

2785-89-9
396 suppliers
$5.00/1g
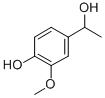
2480-86-6
71 suppliers
$60.00/50mg