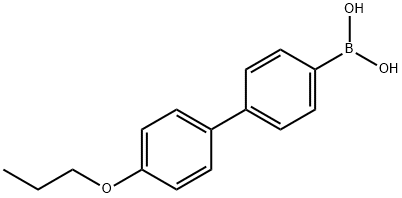
4-(4'-PROPOXYPHENYL)PHENYLBORONIC ACID synthesis
- Product Name:4-(4'-PROPOXYPHENYL)PHENYLBORONIC ACID
- CAS Number:849062-20-0
- Molecular formula:C15H17BO3
- Molecular Weight:256.1
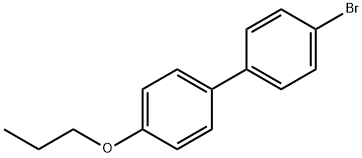
154020-02-7
20 suppliers
$55.00/250mg

849062-20-0
36 suppliers
$60.00/100mg
Yield:849062-20-0 98.4%
Reaction Conditions:
Stage #1: 4-bromo-4'-n-propoxybiphenylwith n-butyllithium in tert-butyl methyl ether at -20; for 2 h;Inert atmosphere;
Stage #2: with Triisopropyl borate in tetrahydrofuran;tert-butyl methyl ether at -60 - 20;
Stage #3: with hydrogenchloride;water in tetrahydrofuran;tert-butyl methyl ether at 20; for 0.166667 h;
Steps:
General procedure: n-BuLi (2.5 M, 7.4 mL, 18 mmol) was added dropwise to a solution of compound 14c (4.5 g, 13 mmol) in MTBE (50 mL) at -20 °C under nitrogen atmosphere. After stirring for 2 h, the reaction mixture was cooled to -60 °C and was added THF (6 mL). Then, a solution of (i-PrO)3B (6.1 mL, 26 mmol) in MTBE (8 mL) was added dropwise to the resulting mixture and was stirred for 1 h. The reaction mixture was allowed to warm to room temperature, stirred overnight and treated with 2 M HCl (50 mL) for 10 min. The organic layer was separated and the solvent was evaporated under reduced pressure. The residue was added hexane (40 mL) and stirred for 10 min. After filtration, the filter cake was washed by hexane-MTBE (8: 1) to give 15c (3.7 g, yield 91.4%) as white solid.
References:
Yao, Jianzhong;Liu, Hongming;Zhou, Ting;Chen, Hai;Miao, Zhenyuan;Sheng, Chunquan;Zhang, Wannian [European Journal of Medicinal Chemistry,2012,vol. 50,p. 196 - 208] Location in patent:supporting information; experimental part
29558-77-8
224 suppliers
$6.00/5g

849062-20-0
36 suppliers
$60.00/100mg
148-86-7
72 suppliers
$11.00/5g

849062-20-0
36 suppliers
$60.00/100mg
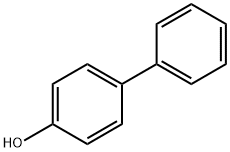
92-69-3
425 suppliers
$6.00/25g

849062-20-0
36 suppliers
$60.00/100mg
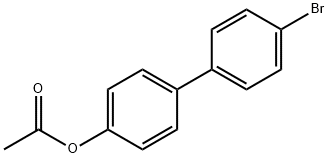
84244-98-4
29 suppliers
$45.00/250mg

849062-20-0
36 suppliers
$60.00/100mg