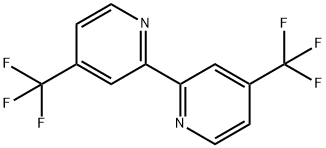
4,4'-bis(trifluoromethyl)-2,2'-bipyridine synthesis
- Product Name:4,4'-bis(trifluoromethyl)-2,2'-bipyridine
- CAS Number:142946-79-0
- Molecular formula:C12H6F6N2
- Molecular Weight:292.18
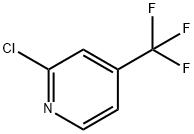
81565-18-6
346 suppliers
$8.00/1g

142946-79-0
63 suppliers
$20.00/250mg
Yield:142946-79-0 89%
Reaction Conditions:
with nickel(II) chloride hexahydrate;triphenylphosphine;zinc in N,N-dimethyl-formamide at 80; for 72 h;Inert atmosphere;Reagent/catalyst;Solvent;Temperature;
Steps:
I. Preparation of 4,4'-bis(trifluoromethyl)-2,2'-bipyridine (2).
In a screw capped tube, NiCl2·6H2O, (238 mg, 1mmol) and PPh3, (524 mg, 2 mmol) were dissolved in 2 mL reagent grade DMF. This blue solution was sparged with argon for 30 min. Activated1 zinc dust (98 mg, 1.5 mmol) was added and the mixture was stirred with argon sparging for 1 h. To the resulting red-brown slurry, 2-chloro-4-(trifluoromethyl)pyridine (1) (183 mg, 1 mmol) was added. The tube was capped and heated in an 80 °C oil bath for 72 h at which time GC-MS showed full consumption of 1. The reaction mixture was then poured into a beaker containing 2 mL ammonia (24% aq) and 20 g ice. The mixture was extracted with diethyl ether. The diethyl ether phase was dried over MgSO4, filtered, and evaporated. The crude product was purified on a silica gel column (4:1 hexane/CH2Cl2) giving 129 mg (89%) of 2 as a white solid; mp 79-80 °C. 1H NMR (500 MHz, CDCl3) δ 8.87 (d, J = 5.0 Hz, 2 H), 8.71 (s, 2 H), 7.57 (d, J = 5.0 Hz, 2 H); 13C NMR (125 MHz, CDCl3) δ 156.1, 150.3, 139.6 (q, J = 34 Hz), 122.8 (q, J = 273 Hz), 119.9 (q, J = 3.5 Hz), 117.1 (q, J = 3.8 Hz); 19F NMR (470 MHz, CDCl3) δ -64.9.
References:
Li, Hao;Oppenheimer, Jossian;Smith, Milton R.;Maleczka, Robert E. [Tetrahedron Letters,2016,vol. 57,# 21,p. 2231 - 2232] Location in patent:supporting information
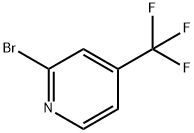
175205-81-9
214 suppliers
$20.00/250 mg

142946-79-0
63 suppliers
$20.00/250mg
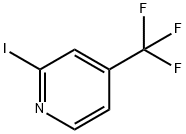
326894-74-0
8 suppliers
inquiry

142946-79-0
63 suppliers
$20.00/250mg
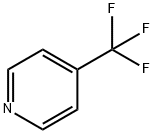
3796-24-5
195 suppliers
$12.00/1g

142946-79-0
63 suppliers
$20.00/250mg