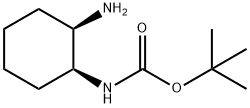
Carbamic acid, [(1S,2R)-2-aminocyclohexyl]-, 1,1-dimethylethyl ester (9CI) synthesis
- Product Name:Carbamic acid, [(1S,2R)-2-aminocyclohexyl]-, 1,1-dimethylethyl ester (9CI)
- CAS Number:365996-30-1
- Molecular formula:C11H22N2O2
- Molecular Weight:214.3
![Carbamic acid, [(1R,2S)-2-aminocyclohexyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/364385-54-6.gif)
364385-54-6
80 suppliers
$36.00/250mg
![Carbamic acid, [(1S,2R)-2-aminocyclohexyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/365996-30-1.gif)
365996-30-1
158 suppliers
$34.00/100mg
Yield:365996-30-1 34%
Reaction Conditions:
Stage #1:(1S,2R)-2-tert-Butoxycarbonylamino-cyclohexylamine with (R)-Mandelic Acid in ethyl acetateLarge scale;
Stage #2: with potassium carbonate in water;ethyl acetate at 20 - 30; for 3 h;Large scale;
Steps:
6.3; 6.4
To a 1000L reactor was added racemate 6.3 (13 1.3kg, 613mo1) in EtOAc and Dmandelic acid (0.5 eq). The reaction mixture was stirred for overnight and then resulting solid was filtered and washed with EtOAc. 96% e.e. of the salt was obtained with 34% yield. The mother liquor was reacted with K2C03 (aq.) and free-diamine was prepared. The free-diamine isolated was reacted with L-mandelic acid. 98% e.e. of the opposite salt 6.5 was obtained with 39% yield. The same operation was repeated four times. 89.8kg of (1S,2R)-salt 6.4 were obtained with high e.e. This salt was recrystallized from isopropanol. 77.7kg of(1S,2R)-salt 6.4 with 98% e.e. was obtained in 69% yield. To the 1000L reactor was added (1S,2R)-salt 6.4, (77.7kg, 212mo1) and ethyl acetate. And then K2C03 aq. (35.1kg, 254mo1) was added. The mixture was stirred for 3hrs at 20-30°C. The organic layer was separated and the aqueous layer was extracted with ethyl acetate. The combined organic extracts were washed with sat. NaC1 aq. The organic layer was dried by Na2504 and then inorganic residue was removed by filtration. The filtrate was concentrated at approximately 40°C under reduced pressure. The crude product (42.1kg) was purified by distillation and the desired product (1S,2R)-N-Boc-1,2-cyclohexanediamine 6.6 (35.4kg, 147-150°C 1-2 mm Hg) was collected. Its purity was 99.5%, 98.0%e.e. by GC analysis.
References:
PORTOLA PHARMACEUTICALS, INC.;PANDEY, Anjali WO2014/152768, 2014, A1 Location in patent:Paragraph 0101; 0102
365996-29-8
2 suppliers
inquiry
![Carbamic acid, [(1S,2R)-2-aminocyclohexyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/365996-30-1.gif)
365996-30-1
158 suppliers
$34.00/100mg
![Carbamic acid, N-[(1S,2S)-2-[(methylsulfonyl)oxy]cyclohexyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/365996-28-7.gif)
365996-28-7
0 suppliers
inquiry
![Carbamic acid, [(1S,2R)-2-aminocyclohexyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/365996-30-1.gif)
365996-30-1
158 suppliers
$34.00/100mg
24424-99-5
832 suppliers
$13.50/25G
![Carbamic acid, [(1S,2R)-2-aminocyclohexyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/365996-30-1.gif)
365996-30-1
158 suppliers
$34.00/100mg
286-20-4
200 suppliers
$14.00/25mL
![Carbamic acid, [(1S,2R)-2-aminocyclohexyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/365996-30-1.gif)
365996-30-1
158 suppliers
$34.00/100mg
