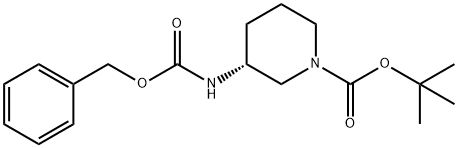
1-Piperidinecarboxylic acid, 3-[[(phenylmethoxy)carbonyl]amino]-, 1,1-dimethylethyl ester, (3R)- synthesis
- Product Name:1-Piperidinecarboxylic acid, 3-[[(phenylmethoxy)carbonyl]amino]-, 1,1-dimethylethyl ester, (3R)-
- CAS Number:320580-76-5
- Molecular formula:C18H26N2O4
- Molecular Weight:334.41
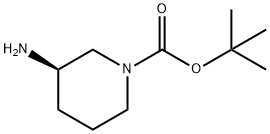
188111-79-7
361 suppliers
$6.00/1g
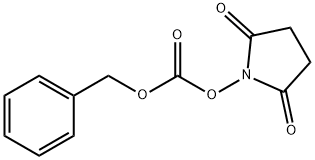
13139-17-8
504 suppliers
$6.00/25g
![1-Piperidinecarboxylic acid, 3-[[(phenylmethoxy)carbonyl]amino]-, 1,1-dimethylethyl ester, (3R)-](/CAS/GIF/320580-76-5.gif)
320580-76-5
43 suppliers
inquiry
Yield:320580-76-5 78.0 %
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in tetrahydrofuran at 25;
Steps:
Step (a): preparation of tert-butyl (3R)-3-(benzyloxycarbonylamino)piperidine-1-carboxylate (compound C1.2)
To a solution of tert-butyl (3R)-3-aminopiperidine-1-carboxylate (compound C1.1, 10.0 g, 49.9 mmol) in THF (200 mL) was added Cbz-OSu (14.9 g, 59.9 mmol) and DIEA (9661.5 mg, 74.9 mmol), the resultant mixture was stirred at 25 °C for 2 hrs. The reaction was quenched with N HCl (aq. 50 mL), and extracted with EA (50 mL) for three times. The combined organic layer was washed with brine, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography (silica gel, 20% to 50% EA in PE) to afford compound C1.2 (13.0 g, 78.0 % yield). MS: calc'd 335 [(M+H)+]; measured 357, [(M+Na)+],
References:
WO2022/243346,2022,A1 Location in patent:Page/Page column 35

188111-79-7
361 suppliers
$6.00/1g

501-53-1
397 suppliers
$10.00/1g
![1-Piperidinecarboxylic acid, 3-[[(phenylmethoxy)carbonyl]amino]-, 1,1-dimethylethyl ester, (3R)-](/CAS/GIF/320580-76-5.gif)
320580-76-5
43 suppliers
inquiry