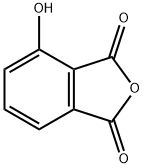
3-Hydroxyphthalic anhydride synthesis
- Product Name:3-Hydroxyphthalic anhydride
- CAS Number:37418-88-5
- Molecular formula:C8H4O4
- Molecular Weight:164.12
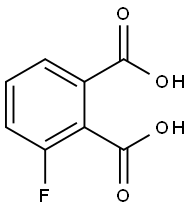
1583-67-1
210 suppliers
$8.00/1g

37418-88-5
259 suppliers
$20.00/250mg
Yield:37418-88-5 88.2%
Reaction Conditions:
Stage #1:3-fluorophthalic acid with copper(l) iodide;potassium hydroxide in N,N-dimethyl-formamide at 100; for 6 h;
Stage #2: with dicyclohexyl-carbodiimide in N,N-dimethyl-formamide at 100; for 2 h;Reagent/catalyst;Solvent;Temperature;
Steps:
1
In3-fluorophthalic acid (184.0 g, 1.0 mol) and N, N-dimethylformamide (600 mL) were added to the three-necked flask,Potassium hydroxide (112.2 g, 2. Omol) and cuprous iodide (5. 7 g, 0.03 mol) were added to the reaction, and the reaction was allowed to proceed at 100 ° C for 6 hours.TLC monitoring (methanol: dichloromethane = 1: 1, v / v) to complete the reaction,And the filtrate was adjusted to pH 1.0 to 2.0 with 6. Omol / L hydrochloric acid. The filtrate was transferred to a three-necked flask and, with stirring, dicyclohexylcarbodiimide (722. 2g, 3.5mol), heated to 100 ° C incubation reaction 2 hours;The organic phase was extracted with acetone (600 mL X 3), dried over anhydrous magnesium sulphate, filtered, filtered, and evaporated to dryness, dried over anhydrous magnesium sulfate,The yield of 3-hydroxyphthalic anhydride was 162.3 g, the conversion was 88.2%, and the HPLC purity was 95.5%.
References:
Jinan Xuan Hong Biological?Pharmaceutial Co.,?Ltd;Liu, Yadong;Li, Zhanjiang;Liu, Caiyun CN105330634, 2016, A Location in patent:Paragraph 0025; 0026
601-97-8
24 suppliers
$155.00/50mg

37418-88-5
259 suppliers
$20.00/250mg
135414-50-5
0 suppliers
inquiry

37418-88-5
259 suppliers
$20.00/250mg
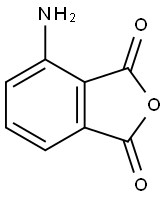
17395-99-2
48 suppliers
inquiry

37418-88-5
259 suppliers
$20.00/250mg
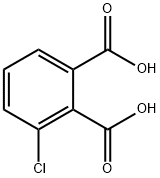
27563-65-1
46 suppliers
$45.00/5g

37418-88-5
259 suppliers
$20.00/250mg