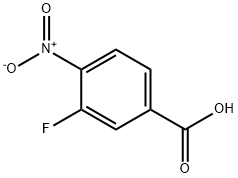
3-Fluoro-4-nitrobenzoic acid synthesis
- Product Name:3-Fluoro-4-nitrobenzoic acid
- CAS Number:403-21-4
- Molecular formula:C7H4FNO4
- Molecular Weight:185.11
446-34-4
303 suppliers
$7.00/5g

403-21-4
254 suppliers
$6.00/1g
Yield:403-21-4 83%
Reaction Conditions:
with potassium dichromate;sulfuric acid in acetic acid at 120; for 2 h;
Steps:
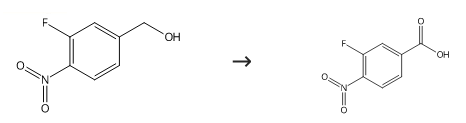
445
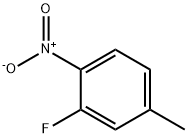
2-Fluoro-4-methyl-l-nitro-benzene (1.0 g, 12.9 mmol) was added portion- wise to a suspension of potassium dichromate (5.04 g, 17.16 mmol) in glacial acetic acid (8 mL) follow by concentrated sulfuric acid (3.6 mL). The reaction mixture was heated to 1200C for 2 h and then allowed to cool to ambient temperature. The reaction was quenched with crushed ice and extracted with ethyl acetate. The organic layer was washed with brine, dried over anhydrous sodium sulfate and concentrated in vacuo to afford 3-fluoro-4-nitro-benzoic acid (1.9 g, 83%) as a white solid.
References:
F2G LTD WO2009/130481, 2009, A1 Location in patent:Page/Page column 145
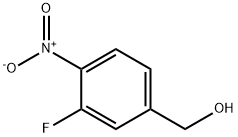
503315-74-0
56 suppliers
inquiry

403-21-4
254 suppliers
$6.00/1g
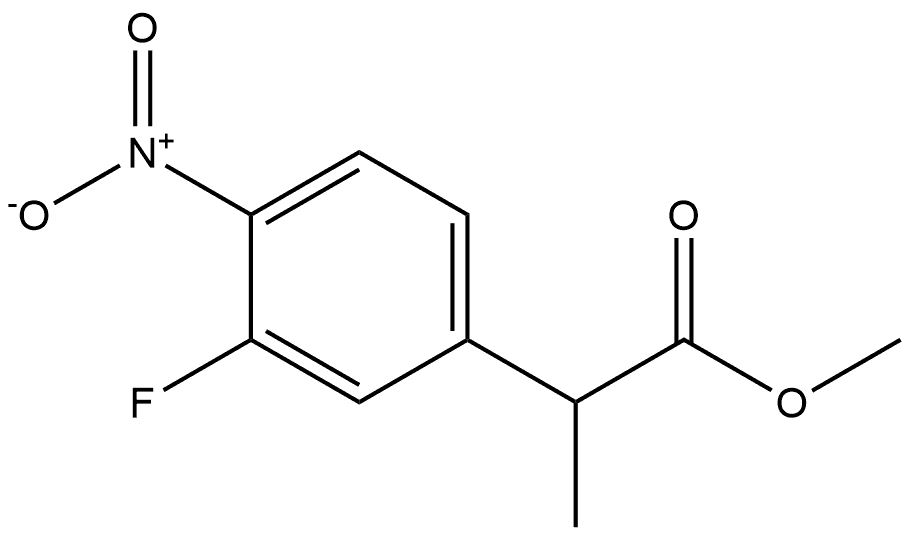
86790-39-8
0 suppliers
inquiry

403-21-4
254 suppliers
$6.00/1g
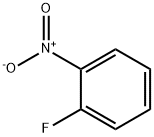
1493-27-2
508 suppliers
$5.00/10g

403-21-4
254 suppliers
$6.00/1g
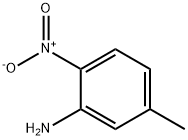
578-46-1
132 suppliers
$6.00/250mg

403-21-4
254 suppliers
$6.00/1g