
3-ACETOXY-5-BROMOINDOLE synthesis
- Product Name:3-ACETOXY-5-BROMOINDOLE
- CAS Number:17357-14-1
- Molecular formula:C10H8BrNO2
- Molecular Weight:254.08
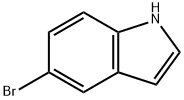
10075-50-0
683 suppliers
$5.00/10g
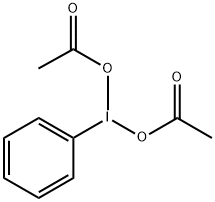
3240-34-4
589 suppliers
$7.00/10g

17357-14-1
125 suppliers
$26.00/100mg
Yield: 99%
Reaction Conditions:
with potassium hydroxide in acetonitrile at 35; for 1.5 h;Inert atmosphere;
Steps:
37 Synthesis of AB17658
As shown in Fig. 133B, a mixture of compound 5a (337 mg, 1.73 mmol, 1.0 eq), compound 5b (2106) (554 mg, 1.73 mmol, 1.0 eq) and potassium hydroxide (1114 mg, 3.46 mmol, 2.0 eq) in acetonitrile (10 mL) was stirred at 35°C for 1.5 h under nitrogen atmosphere. The progress of the reaction mixture was monitored by TLC. After completion of the reaction, the mixture was concentrated under reduced pressure and the residue was purified by silica gel chromatography to afford compound 5c (436 mg, 99%). TLC: PE/EA = 1/1, 254 nm; Rf (Compound 5a) = 0.8; Rf (Compound 5c) = 0.5.
References:
EINZIGER, Michael;SIMPSON, Ann, Marie WO2019/200232, 2019, A1 Location in patent:Paragraph 1219

10075-50-0
683 suppliers
$5.00/10g

17357-14-1
125 suppliers
$26.00/100mg
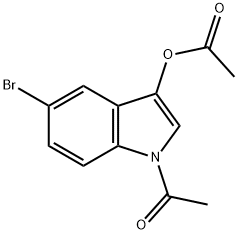
33588-54-4
122 suppliers
$24.00/100mg

17357-14-1
125 suppliers
$26.00/100mg
