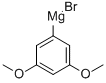
3,5-DIMETHOXYPHENYLMAGNESIUM BROMIDE synthesis
- Product Name:3,5-DIMETHOXYPHENYLMAGNESIUM BROMIDE
- CAS Number:322640-05-1
- Molecular formula:C8H9BrMgO2
- Molecular Weight:241.36
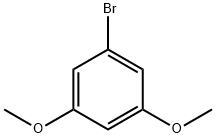
20469-65-2
250 suppliers
$5.00/1g

322640-05-1
13 suppliers
inquiry
Yield:-
Reaction Conditions:
with iodine;magnesium in tetrahydrofuran; for 3 h;Heating / reflux;
Steps:
4.6.2.11
Grignard reagent was prepared in an oven-dried three necked flask outfitted with a reflux condenser, dropping funnel, and magnetic stirrer. 3,5-dimethoxy-bromobenzene (2.0 g, 9.0 mmol) in THF (10 mL) was added to a mixture of magnesium turning (0.2 g, 9.0 mmol) in THF (5 mL) with a small piece of iodine. The resulting mixture was refluxed for about 3 h then cooled to room temperature for about 30 min. The (3,5-dimethoxyphenyl)magnesium bromide was then added slowly to a stirred solution of 1,2-dimethoxy-1H-benzoimidazole-5-carbaldehyde (1.3 g, 7.5 mmol) in THF (10 mL) at 0° C. After complete addition, the solution was allowed to stir at room temperature for about 1 h. The mixture was cooled to 0° C. and quenched with saturated NH4Cl solution (40 mL). The aqueous layer was extracted with EtOAc (3×20 mL). The combined organic layers were washed with water (2×30 mL), brine (30 mL) and dried (MgSO4). Solvent was removed and the crude product was slurried in hexane to afford (3,5-dimethoxy-phenyl)-(1,2-dimethyl-1H-benzoimidazlo-5-yl)-methanol (2.1 g, 91%) as a off white solid: 1H NMR (CDCl3) δ 7.66 (s, 1H), 7.24-7.20 (dd, J=1, 8 Hz, 1H), 7.17 (d, J=8 Hz, 1H), 6.56 (d, J=2 Hz, 2H), 6.31 (t, J=2 Hz, 1H), 5.84 (s, 1H), 3.72 (s, 6H), 3.63 (s, 3H), 3.55 (b, 1H), 2.52 (s, 3H). A suspension of (3,5-dimethoxy-phenyl)-(1,2-dimethyl-1H-benzoimidazol-5-yl)-methanol (2.1 g, 6.7 mmol) and MnO2 (2.9 g, 33.6 mmol) in CH2Cl2 (300 mL) was stirred at room temperature for 17 h. The mixture was filtered through celite and solvent was removed. The crude product was slurried with ether to afford (3,5-dimethoxy-phenyl)-(1,2-dimethyl-1H-benzoimidazol-5-yl)-methanone (2.0 g, 99%) as an off white solid: 1H NMR (DMSO-d6) δ 7.90 (s, 1H), 7.71-7.62 (m, 2H), 6.80 (s, 3H), 3.80 (s, 3H), 3.35 (s, 3H), 2.58 (s, 3H); 13C NMR (DMSO-d6) δ 195.28, 160.17, 154.83, 141.54, 140.27, 139.15, 130.15, 123.50, 120.73, 109.70, 107.19, 103.62, 55.47, 29.96, 13.52. 3-(3,5-Dimethoxy-phenyl)-3-(1,2-dimethyl-1H-benzoimidazol-5-yl)-acrylonitrile (E and Z isomers) were prepared analogously to 3-(3-amino-4-methoxy-phenyl)-3-(3,4-dimethoxy-phenyl)-acrylonitrile (E and Z isomers) using (3,5-dimethoxy-phenyl)-(1,2-dimethyl-1H-benzoimidazol-5-yl)-methanone (2.0 g, 6.4 mmol), lithium bis (trimethylsilyl)amide (7.7 mL, 7.7 mmol) and diethyl cyanomethylphosphate (1.4 g, 7.7 mmol). The crude product was purified by flash chromatography (silica gel, CH2Cl2:CH3OH 95:5) to afford mixture of isomers of 3-(3,5-dimethoxy-phenyl)-3-(1,2-dimethyl-1H-benzoimidazol-5-yl)-acrylonitrile (1.1 g, 50%) as a white solid: mp 199-201° C.; 1H NMR (CDCl3) δ 7.69 (m, 3H), 6.56-6.42 (m, 3H), 5.70 (5.74) (s, 1H), 3.77 (s, 3H), 3.73 (3.74) (s, 6H), 2.61 (s, 3H); 13C NMR (CDCl3) δ 163.76 160.63 (160.60), 153.22 (153.54), 142.35 (142.57), 141.69 (139.43), 137.04 (137.38), 130.69 (132.54), 123.74 (122.64), 120.81 (119.54), 118.23 (118.09), 108.84 (108.80), 107.02 (107.72), 102.08 (102.02), 94.32 (93.47), 55.42 (55.41), 30.05 (30.00), 13.88; Anal Calcd for C20H19N3O2+0.2H2O: C, 71.28; H, 5.80; N, 12.47. Found: C, 71.18; H, 5.86; N, 12.42.
References:
US2006/52596,2006,A1 Location in patent:Page/Page column 43-44
10272-07-8
423 suppliers
$6.00/1g
7787-70-4
441 suppliers
$6.00/25g
7632-00-0
875 suppliers
$14.00/25g

322640-05-1
13 suppliers
inquiry
