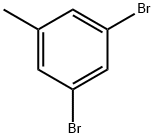
3,5-Dibromotoluene synthesis
- Product Name:3,5-Dibromotoluene
- CAS Number:1611-92-3
- Molecular formula:C7H6Br2
- Molecular Weight:249.93

6968-24-7
210 suppliers
$10.00/5g

1611-92-3
317 suppliers
$6.00/5g
Yield:1611-92-3 93%
Reaction Conditions:
Stage #1:2,6-Dibromo-4-methylaniline with hydrogenchloride;sodium nitrite at 0 - 5; for 0.5 h;
Stage #2: with hydrogenchloride;sodium hypophosphite monohydrate at 0 - 20; for 21 h;
Steps:
1.2 Step 2 The reaction route is:
The specific method is as follows: 1.5L water is sequentially added to the 5L reaction bottle.580 g of hydrochloric acid, cooled to 0-5 ° C in an ice bath,500g of intermediate 1-1 was added in five batches under stirring, and the temperature was controlled at 0-5 ° C.It is a pale yellow suspension. Control temperature does not exceed 5 ° C,Add sodium nitrite solution (156g dissolved in 500mL water),A small amount of reddish brown gas is released and the solid dissolves slowly. After the addition is finished,Stir at 0-5 ° C for 30 minutes, showing a clear liquid with a partial foam on the surface.There is a small amount of black solid on the wall. The hypophosphorous acid solution was added dropwise at 0-5 ° C (mixed with 524 g of sodium hypophosphite monohydrate and 601 g of 30% hydrochloric acid for 1 hour,Filtration to remove undissolved sodium chloride solids),After the completion of the dropwise addition, the mixture was stirred at 0-5 ° C for 5 h, and the ice bath was removed.Slowly rise to 15-20 ° C and stir for 16 h. Sampling HPLC was used to detect the remaining 0.87% of the intermediate diazonium salt, filtered under reduced pressure, and washed with water to obtain 570 g of wet product.Add 800 mL of methanol to the temperature of 50 ° C, stir and melt the layer, stop heating,Stir and cool to 0-10 ° C, suction filtration, get 514g wet product,Drying under reduced pressure at 30 ° C gave 440 g of yellow solid product 1-2,HPLC purity: 94%, yield: 93%,Can be used directly in the next reaction.The nuclear magnetic resonance spectrum of the yellow solid 1-2 is shown in Fig. 2.
References:
Shanghai Qiaokun Chemical Technology Co., Ltd.;Zuo Li;Wang Wenhua;Hu Weijie;Mu Jiapeng CN108250046, 2018, A Location in patent:Paragraph 0053; 0060; 0061; 0062
106-49-0
398 suppliers
$5.00/5 g

1611-92-3
317 suppliers
$6.00/5g
540-23-8
93 suppliers
$20.00/25g

1611-92-3
317 suppliers
$6.00/5g

95-53-4
436 suppliers
$10.00/1g

1611-92-3
317 suppliers
$6.00/5g
626-39-1
468 suppliers
$5.00/5g
917-54-4
173 suppliers
$44.39/50ml

108-86-1
509 suppliers
$10.00/5g
591-17-3
254 suppliers
$10.00/10g

1611-92-3
317 suppliers
$6.00/5g