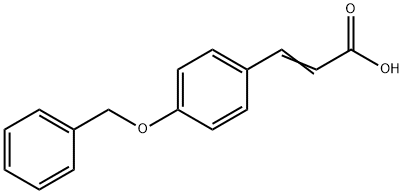
3-[4-(BENZYLOXY)PHENYL]ACRYLIC ACID synthesis
- Product Name:3-[4-(BENZYLOXY)PHENYL]ACRYLIC ACID
- CAS Number:6272-45-3
- Molecular formula:C16H14O3
- Molecular Weight:254.28
![ETHYL 3-[4-(BENZYLOXY)PHENYL]ACRYLATE](/StructureFile/ChemBookStructure4/GIF/CB4167514.gif)
104315-07-3
25 suppliers
$155.00/100mg
![3-[4-(BENZYLOXY)PHENYL]ACRYLIC ACID](/CAS/GIF/6272-45-3.gif)
6272-45-3
74 suppliers
$28.60/100MG
Yield:6272-45-3 100%
Reaction Conditions:
with sodium hydroxide in 1,4-dioxane;water at 20; for 48 h;
Steps:
2 4.1.2 General procedure for the synthesis of cinnamic acid derivatives 35-39 via ethyl cinnamates 30-34
General procedure: To a stirred solution of compounds 25-29 (2.00g), triethyl phosphonoacetate (1.1 equiv.), and potassium carbonate (2.0 equiv.) in dichloromethane/N,N-dimethylformamide (2:1, 15mL) was added a catalytic amount of DBU (0.03 equiv.). The reaction mixture was stirred at room temperature (compounds 25, 27, and 29) or 70°C (26, and 28) for 24-36h. After removal of dichloromethane by evaporation, ice water was added to the reaction mixture which was then stirred for 30min to give precipitates. After filtration, the filter cake was washed with plenty of water, and dried to produce ethyl cinnamates 30-34 as a white or grey solid in yields of 95-98%. The ethyl cinnamates were used directly in the next reaction without characterization. Ethyl cinnamates 30-34 (2.57-4.11g) were added to a 100mL round-bottom flask and 1,4-dioxane (20mL) and 1N-NaOH aqueous solution (8.0-16.0 equiv.) were added subsequently. The reaction mixture was stirred at room temperature for 48h. After completion of the reaction, the reaction mixture was acidified until pH 2 using 2N-HCl aqueous solution. The reaction mixture was stirred at room temperature for 30min and then the resulting solid was filtered. The filter cake was washed with a plenty of water, and dried to give the cinnamic acid derivatives 35-39 as a white or grey solid in yields of 100%.
References:
Ullah, Sultan;Kang, Dongwan;Lee, Sanggwon;Ikram, Muhammad;Park, Chaeun;Park, Yujin;Yoon, Sik;Chun, Pusoon;Moon, Hyung Ryong [European Journal of Medicinal Chemistry,2019,vol. 161,p. 78 - 92]
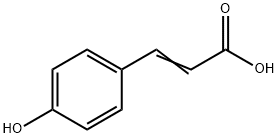
7400-08-0
427 suppliers
$29.00/10g
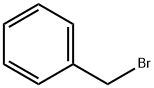
100-39-0
432 suppliers
$10.00/10g
![3-[4-(BENZYLOXY)PHENYL]ACRYLIC ACID](/CAS/GIF/6272-45-3.gif)
6272-45-3
74 suppliers
$28.60/100MG

4397-53-9
321 suppliers
$8.00/10g

141-82-2
749 suppliers
$5.00/25g
![3-[4-(BENZYLOXY)PHENYL]ACRYLIC ACID](/CAS/GIF/6272-45-3.gif)
6272-45-3
74 suppliers
$28.60/100MG
![2-Propenoic acid, 3-[4-(phenylmethoxy)phenyl]-, phenylmethyl ester, (2E)-](/CAS/20210111/GIF/180250-67-3.gif)
180250-67-3
0 suppliers
inquiry
![3-[4-(BENZYLOXY)PHENYL]ACRYLIC ACID](/CAS/GIF/6272-45-3.gif)
6272-45-3
74 suppliers
$28.60/100MG

7400-08-0
427 suppliers
$29.00/10g

100-39-0
432 suppliers
$10.00/10g
![3-[4-(BENZYLOXY)PHENYL]ACRYLIC ACID](/CAS/GIF/6272-45-3.gif)
6272-45-3
74 suppliers
$28.60/100MG