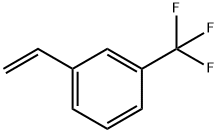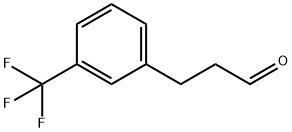
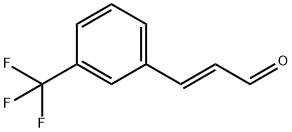
3-(3-(Trifluoromethyl)phenyl)propanal synthesis
- Product Name:3-(3-(Trifluoromethyl)phenyl)propanal
- CAS Number:21172-41-8
- Molecular formula:C10H9F3O
- Molecular Weight:202.17
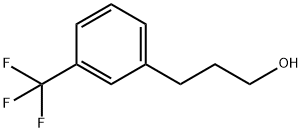
78573-45-2
303 suppliers
$10.00/1g

21172-41-8
183 suppliers
inquiry
Yield:21172-41-8 100%
Reaction Conditions:
Stage #1: 3-[3-(trifluoromethyl)phenyl]propan-1-olwith diphosphorus pentoxide;(methylsulfinyl)methane in dichloromethane at 20;Cooling with ice;Swern Oxidation;
Stage #2: with triethylamine in dichloromethane at 20;Cooling with ice;
Steps:
2
Example 2: Synthesis of 3-[3-(trifluoromethyl)phenyl]-propionaldehyde (II) A solution of compound (V) (1.0 g, 4.90 mmol) in dichloromethane (20 ml) is cooled in water / ice bath, treated in succession with DMSO (770 mg, 9.80 mmol) and P2O5 (1.39 g, 9.80 mmol) and left under stirring for 30 minutes, while temperature raises to 20°C. The reaction mixture is then cooled in water / ice bath and triethylamine (2.4 ml, 17.15 mmol). The resulting solution is kept under stirring while temperature raises to 20°C. After one hour the mixture is treated with 5% HCl, the phases are separated and the organic one is further washed with 5% HCl. The organic phase is then washed with brine, dried over Na2SO4, filtered and concentrated under reduced pressure, to afford 0.99 g of the aldehyde of formula (II) in quantitative yield. 1H NMR (300 MHz, CDCl3), ppm: 9.83 (t, 1H, J 0.9 Hz), 7.48-7.38 (m, 4H), 3.02 (t, 2H, J 7.2 Hz), 2.82 (m, 2H).
References:
EP2327684,2011,A1 Location in patent:Page/Page column 6
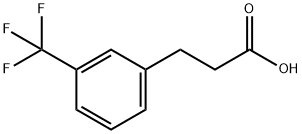
585-50-2
321 suppliers
$20.00/1g

21172-41-8
183 suppliers
inquiry
262268-58-6
98 suppliers
$115.00/5g

21172-41-8
183 suppliers
inquiry
294856-02-3
24 suppliers
inquiry

21172-41-8
183 suppliers
inquiry