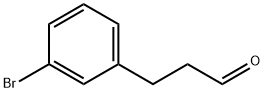
3-(3-BROMO-PHENYL)-PROPIONALDEHYDE synthesis
- Product Name:3-(3-BROMO-PHENYL)-PROPIONALDEHYDE
- CAS Number:210115-30-3
- Molecular formula:C9H9BrO
- Molecular Weight:213.07
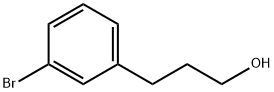
65537-54-4
50 suppliers
$36.00/100mg

210115-30-3
24 suppliers
$110.00/50mg
Yield:210115-30-3 209 mg
Reaction Conditions:
Stage #1: 3-(3-bromophenyl)-propan-1-olwith oxalyl dichloride;dimethyl sulfoxide in tetrahydrofuran at -78; for 1 h;Inert atmosphere;
Stage #2: with triethylamine in tetrahydrofuran at -78; for 0.333333 h;Inert atmosphere;
Steps:
4.1 3-(3-Bromophenyl)propanal (17c)
Borane dimethyl sulfide complex (3.0 mL, 6.1 mmol) was added to a solution of 3-(3-bromophenyl)propionic acid (308 mg, 1.34 mmol) in dry THF (15 mL) at room temperature under inert atmosphere. The mixture was stirred at room temperature for 4.5 h and thereafter diluted with CH2Cl2 and added dropwise to ice-cold 1 M HCl (aq, ~80 mL). NaOH (aq., 10%) was added until pH = 10. The phases were separated and the aqueous phase was extracted with CH2Cl2. The combined organic phases were washed with brine, dried over MgSO4 and concentrated under reduced pressure affording 3-(3-bromophenyl)propanol (282 mg, 98%) as a transparent oil. DMSO (0.28 mL, 3.9 mmol) was added dropwise to a solution of oxalyl chloride (0.14 mL, 1.6 mmol) in dry THF (19 mL) at -78 °C under inert atmosphere. The mixture was stirred for 1 h. A solution of the crude alcohol obtained in the previous step (282 mg, 1.31 mmol) in THF (1.5 mL) was added dropwise to the mixture, which was stirred for 1 h at -78 °C. Et3N (0.91 mL, 6.6 mmol) was added dropwise and stirring was continued for 20 min at -78 °C. Thereafter the mixture was allowed to reach room temperature. Water and EtOAc were added and the phases were separated. The aqueous phase was extracted with EtOAc. The combined organic phases were washed with water and brine, dried over MgSO4, filtered and concentrated under reduced pressure. The crude product was purified by flash column chromatography (10% EtOAc in pentane) affording 17c (209 mg, 73% over two steps) as transparent oil. 1H NMR (400 MHz, CDCl3) δ 9.82 (t, J = 1.2 Hz, 1H), 7.36-7.32 (m, 2H), 7.19-7.10 (m, 2H), 2.97-2.89 (m, 2H), 2.82-2.75 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 200.9, 142.8, 131.5, 130.3, 129.6, 127.2, 122.8, 45.1, 27.8.
References:
Seifert, Tina;Malo, Marcus;Kokkola, Tarja;Stéen, E. Johanna L.;Meinander, Kristian;Wallén, Erik A.A.;Jarho, Elina M.;Luthman, Kristina [Bioorganic and Medicinal Chemistry,2020,vol. 28,# 2,art. no. 115231] Location in patent:supporting information
201230-82-2
1 suppliers
inquiry
2039-86-3
154 suppliers
$15.00/1g

210115-30-3
24 suppliers
$110.00/50mg
59452-90-3
11 suppliers
inquiry
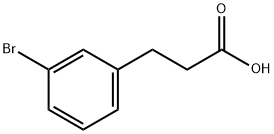
42287-90-1
174 suppliers
$6.00/1g

210115-30-3
24 suppliers
$110.00/50mg

14473-91-7
77 suppliers
$14.00/1g

210115-30-3
24 suppliers
$110.00/50mg
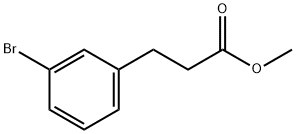
151583-29-8
63 suppliers
$30.00/1g

210115-30-3
24 suppliers
$110.00/50mg