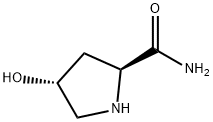
2-Pyrrolidinecarboxamide,4-hydroxy-,(2S,4R)-(9CI) synthesis
- Product Name:2-Pyrrolidinecarboxamide,4-hydroxy-,(2S,4R)-(9CI)
- CAS Number:61703-38-6
- Molecular formula:C5H10N2O2
- Molecular Weight:130.15
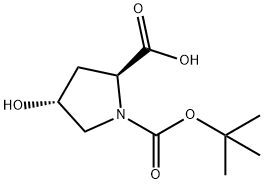
13726-69-7
435 suppliers
$5.00/1g

61703-38-6
27 suppliers
$330.00/250mg
Yield:61703-38-6 55%
Reaction Conditions:
Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acidwith ammonium hydroxide;chloroformic acid ethyl ester;triethylamine in tetrahydrofuran at -17 - 5; for 2 h;
Stage #2: with hydrogenchloride in 1,4-dioxane; for 1 h;
Steps:
3
(4R)-1- (tert-Butoxycarbonyl)-4-hydroxy-L-proline (1.0 g, 4.32 mmol) and triethylamine (0.66 ml, 4.76 mmol) in tetrahydrofuran (15 ml) were cooled TO-15°C. Ethyl chloroformate (0.45 ml, 4.76 mmol) was added drop wise and then concentrated ammonium hydroxide (1.5 ml). The mixture was stirred at 0 to 5°C for 2 hours. Saturated ammonium chloride solution was added and the layers separated. The aqueous layer was re-extracted with tetrahydrofuran and the combined organics dried (MGS04) and concentrated under reduced pressure. Trituration of the residue with ether gave a white solid (610 mg). The solid was stirred in 4M hydrogen chloride in dioxane (10 ML) for 1 hour and then concentrated under reduced pressure. The residue was dissolved in methanol, absorbed onto an ISOLUTE COMMAT; SCX column, washed with methanol and eluted with 7N ammonia in methanol to give (4R)-4-hydroxy-L-prolinamide (320mg, 55%) as a white, crystalline solid; H NMR spectrum : (DMSO d6) 1.67 (ddd, 1H) 1.90 (qt, 1H), 2.70 (dt, 1H), 2. 86 (dd, 1H), 3.63 (t, 1H), 4.16 (m, 1H), 4.63 (brs, 1H), 6.94 (brs, 1H), 7.34 (brs, 1H).
References:
WO2005/26156,2005,A1 Location in patent:Page/Page column 94-95
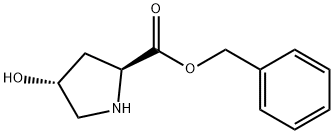
68172-39-4
3 suppliers
inquiry

61703-38-6
27 suppliers
$330.00/250mg
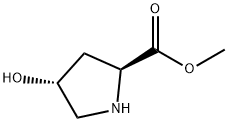
1499-56-5
41 suppliers
$40.00/100mg

61703-38-6
27 suppliers
$330.00/250mg

51-35-4
855 suppliers
$6.00/5g

61703-38-6
27 suppliers
$330.00/250mg