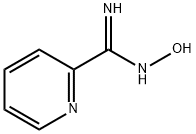
2-Pyridylamid oxime synthesis
- Product Name:2-Pyridylamid oxime
- CAS Number:1772-01-6
- Molecular formula:C6H7N3O
- Molecular Weight:137.14
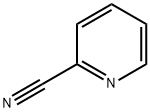
100-70-9
417 suppliers
$13.00/25g

1772-01-6
132 suppliers
$10.00/1g
Yield:1772-01-6 100%
Reaction Conditions:
with hydroxyamino hydrochloride;triethylamine in ethanol; for 3 h;Reflux;
Steps:
Amidoxime Intermediate
2-Pyridinecarboximidamide, N-Hydroxy-
Amidoxime Intermediate
To a 1 L single neck round-bottomed flask equipped with a stir bar were charged 40 g (384 mmol) of 2-pyridinecarbonitrile, 26.7 g (384 mmol) of hydroxylamine hydrochloride, 107 mL (768 mmol) of triethylamine and 300 mL of ethanol.
The flask was fitted with a reflux condenser and the mixture heated under reflux for three hours (TLC showed no remaining starting material).
After concentration on a rotary evaporator, the residue was slurried in a mixture of 300 mL of dichloromethane and 300 mL of water.
The slurry was filtered and the product cake washed several times with water.
The organic layer washed with 2*300 mL of water.
The aqueous layers were backwashed with 50 mL of dichloromethane and the combined organic layers dried over magnesium sulfate, filtered and rotary evaporated, giving a white solid.
The solids were combined and dried in a reduced pressure oven, giving 52.5 g (100%) of product, m.p. 116° C. 1H NMR (400 MHz, DMSO-d6) 9.9 (s, 1H, -OH), 8.53 (m, 1H, Ar-H), 7.85 (d, 1H, Ar-H), 7.78 (t, 1H, Ar-H), 7.38 (m, 1H, Ar-H), 5.82 (br s, 2H, -NH2), 1.90-0.85 (m, 19H, aliphatics).
References:
US2016/244860,2016,A1 Location in patent:Paragraph 0144-0145
2739-97-1
217 suppliers
$10.00/1g

1772-01-6
132 suppliers
$10.00/1g

1121-60-4
509 suppliers
$10.60/10gm:

1772-01-6
132 suppliers
$10.00/1g

873-69-8
185 suppliers
$8.00/5g

1772-01-6
132 suppliers
$10.00/1g