
2-Phenylethylamine hydrochloride synthesis
- Product Name:2-Phenylethylamine hydrochloride
- CAS Number:156-28-5
- Molecular formula:C8H12ClN
- Molecular Weight:157.64
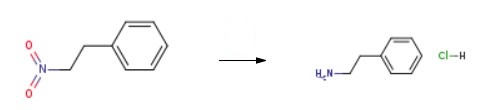
63-91-2
874 suppliers
$10.00/100G

156-28-5
266 suppliers
$21.00/100g
Yield:156-28-5 78%
Reaction Conditions:
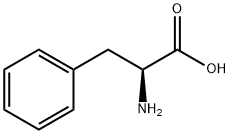
Stage #1: L-phenylalaninewith (R)-Carvone in propan-1-ol at 190; under 11251.1 Torr; for 0.0833333 h;Sealed tube;
Stage #2: with hydrogenchloride in propan-1-ol;water at 190; for 0.0833333 h;
Steps:
General “One-Pot” Procedure for the Decarboxylation of Amino Acids
General procedure: General “One-Pot” Procedure for the Decarboxylation of Amino Acids [0045] A magnetic stir bar, 3 mL of n-PrOH, 10 mmol of R-Carvone, and 5 mmol of amino acid were charged to a pressure vessel. The vessel was heated from room temperature to 190° C. over 5 min with stirring. If necessary the reaction vessel was maintained at 190° C. for additional time until the slurry became clear. The vessel was allowed to cool to below the solvent boiling point, carefully vented to release evolved CO2, and 10 mL of 2M HCl was added. The vessel was heated to 190° C. over 5 min with stirring and allowed to cool. The aqueous reaction mixture was washed three times with 25 mL of ether and water solvent distilled off from the hydrochloride salt. The hydrochloride salt was transferred to a vacuum oven and dried overnight at 150° C. and 10 Torr. The hydrochloride salt was then weighed and analyzed via IR and NMR.; 2-phenylethylamine hydrochloride δH 2.82 2H t J=8, 3.10 2H t J=8, 7.12-7.26 5H m; δC 32.73, 40.55, 127.30, 128.88, 163.03, 170.53
References:
US2014/275569,2014,A1 Location in patent:Page/Page column 0045; 0057; 0066; 0067
140-29-4
5 suppliers
$19.00/25mL

156-28-5
266 suppliers
$21.00/100g
6125-24-2
58 suppliers
$183.15/1g

156-28-5
266 suppliers
$21.00/100g
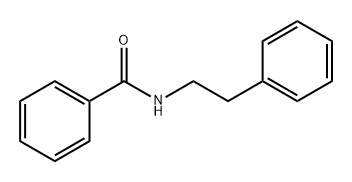
3278-14-6
114 suppliers
$14.00/1g
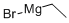
925-90-6
343 suppliers
$12.00/5ml

156-28-5
266 suppliers
$21.00/100g

93-55-0
7 suppliers
$15.00/25g
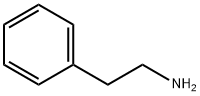
64-04-0
8 suppliers
$10.00/5mL

156-28-5
266 suppliers
$21.00/100g