2-METHOXYBIPHENYL synthesis
- Product Name:2-METHOXYBIPHENYL
- CAS Number:86-26-0
- Molecular formula:C13H12O
- Molecular Weight:184.23
Yield: 96%
Reaction Conditions:
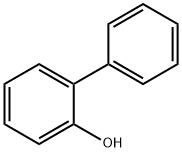
Stage #1:2-Phenylphenol with potassium carbonate for 0.25 h;Inert atmosphere;
Stage #2:dimethyl sulfate in acetone at 20 - 75; for 16 h;Inert atmosphere;
Steps:
2-Phenylanisole (2b).
An oven-dried 500 mL round-bottomed two necked flask that was equipped with a magnetic stirring bar, dropping funnel and reflux condenser was charged with 2-phenylphenol (7b) (17.0 g, 0.1 mol, 1.00 equiv) and potassium carbonate (19.4 g,0.14 mol, 1.40 equiv). The flask was evacuated for 15 minutes, back-filled with dry N2, andglass stopper of dropping funnel replaced with a rubber septum under positive pressure of dry nitrogen. 2-Phenylphenol was dissolved by adding dried acetone (250 mL). Then, dimethyl sulfate (12.3 mL,16.4 g, 0.13 mol, 1.30 equiv) was dropwise added into the reaction mixture at room temperature, by means of a dropping funnel. After the addition of dimethyl sulfate was completed, the reaction flask was heated to 75 °C and stirred for 16 h at this temperature. The conversion was followed by TLC. After the conversion was completed, the flask was cooled down to room temperature, solids were removed by filtration, and the filtrate was concentrated by rotary evaporation in vacuo. Purification of the crude product by flash column chromatography on silica gel(hexanes/EtOAc, 10:0.5) afforded 17.70 g (96.0 mmol, 96%) of the title compound (2b) as a colorless oil. TLC: Rf= 0.84 (silica gel; hexanes/EtOAc, 10:0.5). 1H NMR (500 MHz, CDCl3): = 3.90 (d, J = 0.9 Hz, 3H), 7.13 (ddd, J =28.4, 17.4, 4.6 Hz, 2H), 7.40-7.47 (m, 3H), 7.48-7.56 (m, 2H), 7.63-7.70 (m, 2H).
References:
Dilek, ?mer;Tezeren, Mustafa A.;Tilki, Tahir;Ertürk, Erkan [Tetrahedron,2018,vol. 74,# 2,p. 268 - 286] Location in patent:supporting information