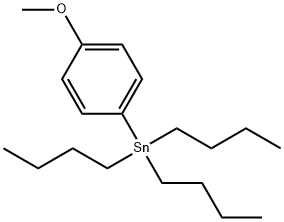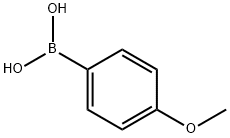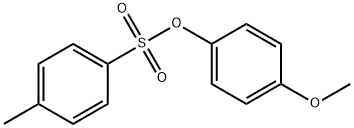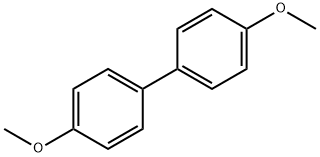
4,4'-Dimethoxybiphenyl synthesis
- Product Name:4,4'-Dimethoxybiphenyl
- CAS Number:2132-80-1
- Molecular formula:C14H14O2
- Molecular Weight:214.26
Yield: 97%
Reaction Conditions:
with 4,4’‐bis(trimethylammoniummethyl)‐2,2’‐bipyridine;diamminedichloropalladium(II);sodium hydrogencarbonate in water at 110; for 36 h;Sealed tube;Stille Cross Coupling;
Steps:
3.2. Typical Stille Coupling Procedure
General procedure: A sealable tube, equipped with a magnetic stirring bar, was charged with aryl halide (1 mmol), organotin (1.2 mmol), NaHCO3 (2 mmol), and H2O (2 mL). In the case of tetramethyltin, the addition of tetraethylammonium iodide (TEAI, 1 mmol) was required. After the addition of PdCl2(NH3)2/L aqueous solution (1 mL H2O; different concentrations were required for various substrate/catalystratios), the tube was sealed under air using a Teflon-coated screw cap. The reaction vessel was then placed in an oil bath at 110 °C for the indicated reaction duration (see Tables 2-4). After cooling of the reaction mixture to room temperature, the aqueous solution was extracted with hexane or ethyl acetate; the organic phase was dried over MgSO4, and the solvent was then removed under vacuum. Column chromatography on silica gel afforded the desired product (see Supplementary Materials for the copies of NMR spectra).
References:
Wu, Wei-Yi;Liu, Ling-Jun;Chang, Fen-Ping;Cheng, Yu-Lun;Tsai, Fu-Yu [Molecules,2016,vol. 21,# 9,art. no. 1205]

104-92-7
430 suppliers
$10.00/10g
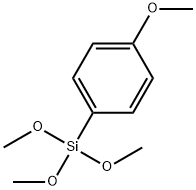
35692-27-4
61 suppliers
$309.00/5g

2132-80-1
91 suppliers
$11.00/5g
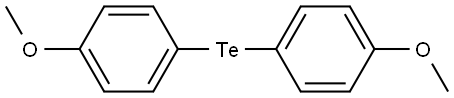
4456-34-2
2 suppliers
inquiry

2132-80-1
91 suppliers
$11.00/5g