
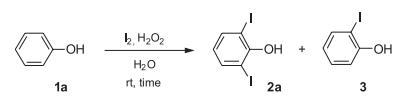
2-Iodophenol synthesis
- Product Name:2-Iodophenol
- CAS Number:533-58-4
- Molecular formula:C6H5IO
- Molecular Weight:220.01
1008106-86-2
56 suppliers
$72.00/500mg

533-58-4
372 suppliers
$6.00/5g
Yield:533-58-4 93%
Reaction Conditions:
with [bis(acetoxy)iodo]benzene;water;triethylamine in acetonitrile at 20; for 0.166667 h;
Steps:
General procedure for syntheses of aromatic alcohols
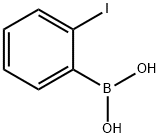
General procedure: To a stirred solution of appropriate organoboronic acids (0.5 mmol, 1.0 equiv.) and Et3N(1.0 mmol, 2.0 equiv.) in CH3CN(acetonitrile: 3 mL, H2O: 11μL, 0.6mmol, 1.2 equiv.), DAIB (0.75 mmol, 1.5 equiv.), dissolved in acetonitrile (2mL) was added drop wise at room temperature and the mixture was allowed to stir for 10 minutes at that temperature. After completion of the reaction indicated by TLC, the reaction mixture was washed with distilled water (3×7 mL) and extracted with CH2Cl2(3×10 mL). The combined organic phase was dried over Na2SO4 and after evaporating the solvent, the residue was purified by column chromatography over silica gel using hexane/EtOAc as eluent to provide the pure target product.
References:
Chatterjee, Nachiketa;Chowdhury, Hrishikesh;Sneh, Kumar;Goswami, Avijit [Tetrahedron Letters,2014,vol. 56,# 1,p. 172 - 174] Location in patent:supporting information
24892-63-5
4 suppliers
inquiry

533-58-4
372 suppliers
$6.00/5g
108-95-2
744 suppliers
$14.00/25g

533-58-4
372 suppliers
$6.00/5g
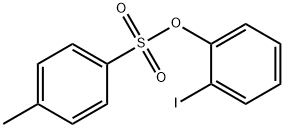
136859-32-0
7 suppliers
inquiry

533-58-4
372 suppliers
$6.00/5g
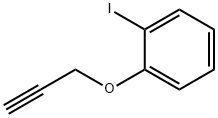
41876-99-7
4 suppliers
inquiry

533-58-4
372 suppliers
$6.00/5g