
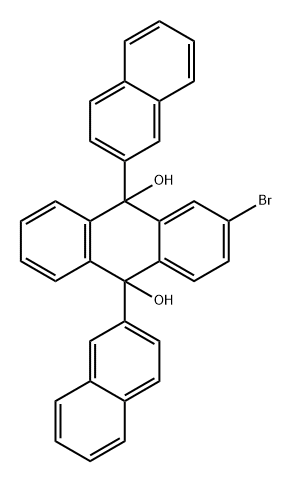
2-Bromo-9,10-bis(2-naphthalenyl)anthracene synthesis
- Product Name:2-Bromo-9,10-bis(2-naphthalenyl)anthracene
- CAS Number:474688-76-1
- Molecular formula:C34H21Br
- Molecular Weight:509.43
474688-75-0
1 suppliers
inquiry

474688-76-1
220 suppliers
$11.00/250mg
Yield:474688-76-1 96%
Reaction Conditions:
with sodium hypophosphite;potassium iodide in acetic acid; for 3 h;Heating / reflux;
Steps:
9
Synthesis of Compound 1092-Bromonaphthalene (11.0 g, 53.1 mmol) was dissolved in dry tetrahydrofuran (100 mL) under a nitrogen atmosphere at room temperature. The solution was cooled to -78° C. in a cooling bath, and t-butyllithium (47.0 mL, 1.7 pentane solution) was slowly added thereto. The mixture was stirred at the same temperature for about 1 hour, and Compound 104 (6.31 g, 22.0 mmol) was added thereto still at the same temperature. Then the cooling bath was removed, and the mixture was stirred at room temperature for about 3 hours. To the stirred mixture an aqueous ammonium chloride solution was added. The resulting mixture was extracted with methylene chloride. The organic extract was dried over magnesium sulfate and concentrated under reduced pressure. The crude product was dissolved in diethyl ether, and then petroleum ether was added thereto. The mixture was agitated for several hours to obtain a solid compound. The solid was filtered and vacuum dried to obtain dinaphthyl dialcohol (11.2 g, 93%). The dinaphtyl dialcohol (11.2 g, 20.5 mmol) was dispersed in 600 mL of acetic acid under a nitrogen atmosphere, to which potassium iodide (34.2 g, 206 mmol) and sodium hypophosphite hydrate (36.0 g, 340 mmol) were added. The resulting mixture was agitated while boiling for about 3 hours. After cooling to room temperature, the mixture was filtered and washed with water and methanol, and then vacuum dried to obtain Compound 109 (10.1 g, 96%) a light yellow color.
References:
US7485733,2009,B2 Location in patent:Page/Page column 76
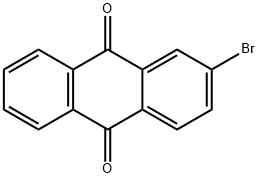
572-83-8
209 suppliers
$6.00/1g

474688-76-1
220 suppliers
$11.00/250mg