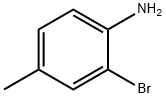
2-Bromo-4-methylaniline synthesis
- Product Name:2-Bromo-4-methylaniline
- CAS Number:583-68-6
- Molecular formula:C7H8BrN
- Molecular Weight:186.05
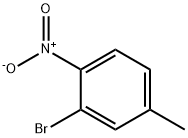
40385-54-4
77 suppliers
$15.00/250mg

583-68-6
290 suppliers
$10.00/5 g
Yield:583-68-6 82%
Reaction Conditions:
with carbon monoxide;triethylamine in tetrahydrofuran;water at 125; for 24 h;Autoclave;
Steps:
Hydrogenation of Nitroarenes 1; General Procedure
General procedure: An 8 mL glass vial (: 14 mm, height 50 mm) equipped with a Tefloncoated oval magnetic stirring bar (8 5 mm) and a plastic screw cap was charged with the corresponding nitroarene 1 (0.5 mmol, 1.0 equiv), Fe2O3/NGr(at)C catalyst (50 mg, 4.0 mol% Fe), Et3N (70 μL, 0.5 mmol, 1.0 equiv), THF (2 mL), and deionized H2O (0.2 mL). The silicone septum was punctured with a 26 gauge syringe needle (0.45 12 mm) and the vial was placed in an aluminum plate, which was then transferred into the 300 mL autoclave. Once sealed, the autoclave was placed into an aluminum block and purged 3 times with CO (at 5-10 bar). Then it was pressurized with CO to 30 bar, followed by additional 20 bar of N2. The aluminum block was heated up to 125 °C under thorough stirring (700 rpm). After 24 h, the autoclave was removed from the aluminum block and cooled to r.t. in a water bath. The remaining gases were discharged and the vials containing reaction products were removed from the autoclave. The reaction mixture was filtered through a Celite pad (~1 cm), concentrated, and analyzed by GC and NMR spectroscoopy (Table 2).
References:
Ryabchuk, Pavel;Junge, Kathrin;Beller, Matthias [Synthesis,2018,vol. 50,# 22,p. 4369 - 4376] Location in patent:supporting information
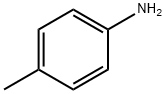
106-49-0
402 suppliers
$5.00/5 g

583-68-6
290 suppliers
$10.00/5 g

106-49-0
402 suppliers
$5.00/5 g

583-68-6
290 suppliers
$10.00/5 g

6968-24-7
211 suppliers
$10.00/5g
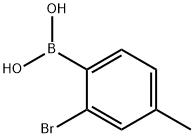
854636-01-4
19 suppliers
inquiry

583-68-6
290 suppliers
$10.00/5 g
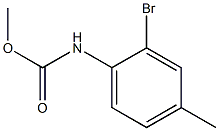
540797-44-2
6 suppliers
inquiry

583-68-6
290 suppliers
$10.00/5 g