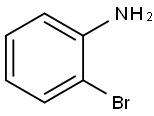
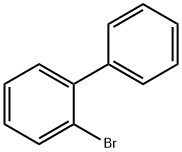
2-Aminobiphenyl synthesis
- Product Name:2-Aminobiphenyl
- CAS Number:90-41-5
- Molecular formula:C12H11N
- Molecular Weight:169.22
NEW SYNTHESIS OF 2-AMINOBIPHENYLS
Reflux 2-nitrobiphenyl, ethanol and stannous chloride in a water bath for 3h, recover the ethanol, cool the residue, add 40% liquid alkali to make it strongly alkaline, separate the oil layer, extract the aqueous layer with ether, dry the extract with anhydrous calcium chloride, filter and recover the ether, wash with ethanol to obtain the crude product, then distill the crude product under reduced pressure to obtain 2-aminobiphenyl.
Yield:90-41-5 98%
Reaction Conditions:
with C21H28N2;potassium carbonate;palladium dichloride in water;N,N-dimethyl-formamide at 60; for 6 h;Inert atmosphere;Suzuki-Miyaura Coupling;
Steps:
2.3 General procedure for Suzuki-Miyaura reaction
General procedure: In a round bottle, ligand or palladium complexes (1 mol% mmol), aryl halides (1.0 mmol), arylboronic acid (1.2 mmol), K2CO3 (2.0 mmol) and 4 mL of DMF and 1 mL of H2O were added with a magnetic stir bar. The reaction mixture was carried out at the described temperature for the required time, and then the solvent was removed under reduced pressure. The residual was diluted with Et2O (5 mL), followed by extraction twice (2 × 5mL) with Et2O. The organic layer was dried with anhydrous MgSO4, filtered and evaporated under vacuum. The crude products were purified by silica-gel column chromatography using petroleum ether-ethyl acetate (20/1) as an eluent, and the isolated yield was then calculated based on the feeding of the aryl halide. The isolated corresponding products were characterized by 1H NMR and 13C NMR.
References:
Cao, Gao;Yang, Hai-Qing;Luo, Bao-Tian;Liu, Feng-Shou [Journal of Organometallic Chemistry,2013,vol. 745-746,p. 158 - 165]
2052-07-5
387 suppliers
$16.30/1g

90-41-5
353 suppliers
$16.00/5g