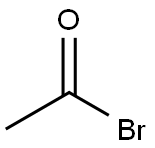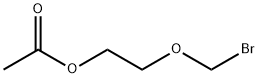
(2-ACETOXYETHOXY)-METHYL BROMIDE synthesis
- Product Name:(2-ACETOXYETHOXY)-METHYL BROMIDE
- CAS Number:81777-40-4
- Molecular formula:C5H9BrO3
- Molecular Weight:197.03
Yield:81777-40-4 100%
Reaction Conditions:
at 0 - 20;
Steps:
(2-acetoxyethoxy)methyl bromide (14)4. Robins, M.J.; Hatfield, P.W. Can. J. Chem. 1982, 60, 547.
Acetyl bromide (13.0 g, 106 mmol) was stirred magnetically with cooling in an ice bath while 7.4 g (100 mmol) of 1, 3-dioxane was added slowly. This solution was stirred for 2 hours at room temperature, giving quantitative conversion to compound 14. The compound was obtained as colourless oil. 1H-NMR (200MHz, DMSO-d6) d: 2.01 (3H, s, CH3), 3.65 (2H, m, CH2), 4.05 (2H, m, CH2), 5.38 (1H, s, OCH2N). ESI MS: m/z 198.1 Da [M+H]+, C5H9BrO3 Mol. Wt. 197.03.
References:
Vertuani, Silvia;Baldisserotto, Anna;Varani, Katia;Borea, Pier Andrea;De Marcos Maria Cruz, Bonache;Ferraro, Luca;Manfredini, Stefano;Dalpiaz, Alessandro [European Journal of Medicinal Chemistry,2012,vol. 54,p. 202 - 209] Location in patent:supporting information; experimental part