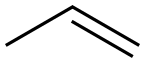
2,3-Dimethylpentane synthesis
- Product Name:2,3-Dimethylpentane
- CAS Number:565-59-3
- Molecular formula:C7H16
- Molecular Weight:100.2
67-56-1
752 suppliers
$7.29/5ml-f
107-83-5
226 suppliers
$10.00/1g

108-08-7
75 suppliers
$47.29/2g
591-76-4
72 suppliers
$85.00/5mL

565-59-3
83 suppliers
$44.20/5g
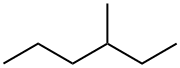
589-34-4
76 suppliers
$95.79/5g

75-28-5
124 suppliers
$40.00/1g

78-78-4
269 suppliers
$20.00/25ML
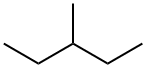
96-14-0
107 suppliers
$22.19/5ml

79-29-8
137 suppliers
$20.00/1g
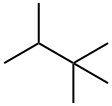
464-06-2
92 suppliers
$23.50/1g
Yield:-
Reaction Conditions:
indium (III) iodide at 180; for 0.5 h;
Steps:
23
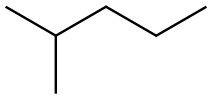
In order to demonstrate that InI3 catalyzed alkane homologation can be used to homologate C6 alkanes other than 2,3-dimethylbutane, a reaction was performed using 2-methylpentane as the starting alkane. The reaction was performed using 6.2 mmol of MeOH, 6.2 mmol of 2-methylpentane, 4.13 mmol of InI3 and 0.367 mmol of adamantane. A similar procedure to that described above for 2,3-dimethylbutane was used and the reaction was heated at 180 0C for 30 minutes. GC analysis indicated that at the end of the reaction 27.4 mg of 2,3-dimethylpentane was formed and 267 mg of 2-methylpentane were recovered. A breakdown of the product distribution is shown in Table 8.Table 8: Products from the homologation of 2-methylpentane with MeOH.Product Yield (mg)Iso-butane 23.5Iso-pentane 10.72,3-dimethylbutane 1.762-methylpentane 2673-methylpentane 73.02,4-dimethypentane 12.1Triptane 5.032-methylhexane 5.252,3-dimethylpentane 27.43-methylhexane 8.48Cs alkanes 17.3[0088] The reaction is presumed to initially result in the methylation of 2- methylpentane to 2,3-dimethylpentane. The mechanism is proposed to be analogous to that described above for 2,3-dimethylbutane; initial abstraction of a hydride from 2- methylpentane to form the tertiary 2-methylpentyl carbocation, deprotonation to give 2-methyl-2-pentene, methylation to give the most substituted carbocation, in this case the tertiary 2, 3-dimethylpentyl carbocation, which subsequently picks up a hydride to form 2,3-dimethylpentane. Under the reaction conditions, 2,3- dimethylpentane is unstable and undergoes both isomerization and cracking reactions to form some of the other products observed. Isomerization of 2,3-
References:
CALIFORNIA INSTITUTE OF TECHNOLOGY;BP P.L.C. WO2009/64622, 2009, A2 Location in patent:Page/Page column 27-28

75-28-5
124 suppliers
$40.00/1g
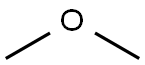
115-10-6
95 suppliers
$75.00/25 g
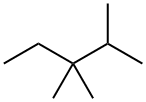
560-21-4
27 suppliers
inquiry
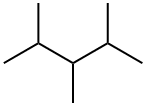
565-75-3
73 suppliers
$52.00/5g

540-84-1
379 suppliers
$16.00/25ML

565-59-3
83 suppliers
$44.20/5g

78-78-4
269 suppliers
$20.00/25ML
67-56-1
752 suppliers
$7.29/5ml-f

108-08-7
75 suppliers
$47.29/2g

565-59-3
83 suppliers
$44.20/5g

75-28-5
124 suppliers
$40.00/1g

78-78-4
269 suppliers
$20.00/25ML

96-14-0
107 suppliers
$22.19/5ml

107-83-5
226 suppliers
$10.00/1g

79-29-8
137 suppliers
$20.00/1g

464-06-2
92 suppliers
$23.50/1g

95-93-2
217 suppliers
$20.00/25g
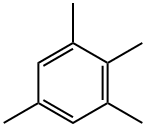
527-53-7
81 suppliers
$127.00/5mL
700-12-9
157 suppliers
$21.00/5g
87-85-4
172 suppliers
$25.19/1g
82166-21-0
0 suppliers
inquiry

108-08-7
75 suppliers
$47.29/2g

565-59-3
83 suppliers
$44.20/5g
617-78-7
32 suppliers
$60.00/100mg