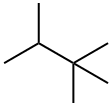
2,2,3-TRIMETHYLBUTANE synthesis
- Product Name:2,2,3-TRIMETHYLBUTANE
- CAS Number:464-06-2
- Molecular formula:C7H16
- Molecular Weight:100.2
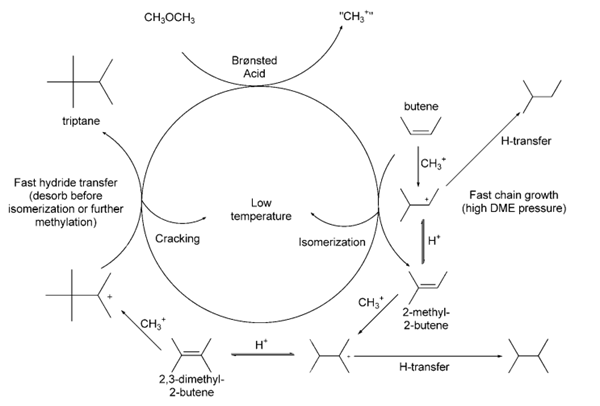
We report herein the first selective catalytic conversion of DME to triptane on halide-free catalysts, specifically on crystalline solid acids, at much lower reaction temperatures (453–493 K) and higher DME pressures (60–250 kPa) than in established methanol/DME to hydrocarbon processes. Low temperatures avoid intervening skeletal isomerization and b-scission of triptyl chains or their precursors. These processes would otherwise occur before the methylation events that form the C7 chains containing the triptane backbone, which desorb irreversibly as triptane.
Yield:464-06-2 56%
Reaction Conditions:
with adamantane;indium (III) iodide at 180; for 0.5 h;Product distribution / selectivity;
Steps:
19
Table 4 compares the yield and selectivity of reactions performed in the presence and absence of adamantane. The triptane yield is based on total converted carbon, while the triptane selectivity is based on the formation of triptane from 2,3- dimethylbutane.Table 4: Comparison of triptane yield and selectivity for homologation in the presence and absence of adamantane.aAdamantane Recovered yield Triptane yield (%)c Triptane selectivity (mg) of 2,3- (%)d dimethylbutane/o/506 51 56 65 0 63 31 39 aUnless otherwise specified, reactions were performed at 180 0C for 30 minutes and used 4.13 mmol of InI3, 6.2 mmol of 2,3-dimethylbutane and 6.2 mmol of MeOH. bAround 6 mol% of adamantane relative to 2,3-dimethylbutane. cBased on total converted carbon. dPercentage of converted 2,3-dimethylbutane which becomes triptane.
References:
WO2009/64622,2009,A2 Location in patent:Page/Page column 20-21
594-83-2
39 suppliers
inquiry

464-06-2
92 suppliers
$26.03/1g
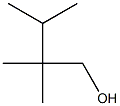
55505-23-2
4 suppliers
inquiry

464-06-2
92 suppliers
$26.03/1g
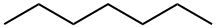
142-82-5
541 suppliers
$17.00/25ML

464-06-2
92 suppliers
$26.03/1g