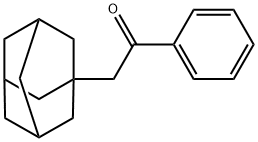
2-(1-ADAMANTYL)-1-PHENYLETHANONE synthesis
- Product Name:2-(1-ADAMANTYL)-1-PHENYLETHANONE
- CAS Number:27648-26-6
- Molecular formula:C18H22O
- Molecular Weight:254.37
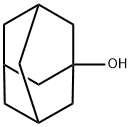
13735-81-4
114 suppliers
$23.52/1g
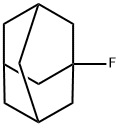
768-92-3
39 suppliers
$45.00/10mg

27648-26-6
9 suppliers
$583.00/1g
Yield:27648-26-6 94%
Reaction Conditions:
with tris(pentafluorophenyl)borane monohydrate in nitromethane at 23; for 1 h;Sealed tube;
Steps:
General Procedure B
General procedure: To a 2 mL disposable vial containing the requisite aliphatic fluoride 1 (0.25 mmol, 1 equiv) in nitromethane (0.125 mL, 2.0M) was added the requisite nucleophile (0.50 mmol, 2 equiv), followed by B(C6F5)3*H2O (1.3 mg, 2.5 μmol, 1.0 mol%). The vial was capped and the mixture allowed to stir for 1 h at room temperature (23 °C), unless another temperature is specified in the procedure. At completion, the reaction mixture was directly dry loaded onto silica and purified by column chromatography over silica according to the conditions given. For reactions at elevated temperatures, an identical procedure was followed, however the reaction was performed in a 10 mL screw-top vial. The desired temperature was achieved using a heating block and contact thermometer.
References:
Dryzhakov, Marian;Richmond, Edward;Li, Guang;Moran, Joseph [Journal of Fluorine Chemistry,2017,vol. 193,p. 45 - 51]

13735-81-4
114 suppliers
$23.52/1g
![Tricyclo[3.3.1.13,7]decane-1-carboxylic acid, 1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl ester](/CAS/20210111/GIF/118334-83-1.gif)
118334-83-1
8 suppliers
inquiry

27648-26-6
9 suppliers
$583.00/1g

768-90-1
407 suppliers
$10.00/5g

13735-81-4
114 suppliers
$23.52/1g

27648-26-6
9 suppliers
$583.00/1g