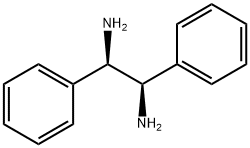
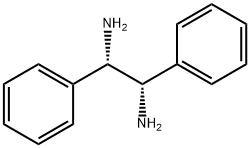
(1R,2R)-(+)-1,2-Diphenylethylenediamine synthesis
- Product Name:(1R,2R)-(+)-1,2-Diphenylethylenediamine
- CAS Number:35132-20-8
- Molecular formula:C14H16N2
- Molecular Weight:212.29

16118-22-2
3 suppliers
inquiry

35132-20-8
361 suppliers
$7.00/1g
Yield:35132-20-8 82%
Reaction Conditions:
Stage #1: benzylidenaminewith (5R,5'R)-4,4,4',4'-tetrakis(2,5-dimethylphenyl)-5,5'-diphenyl-2,2'-bi(1,3,2-dioxaborolane) in tetrahydrofuran;methanol at 20 - 30;Schlenk technique;Inert atmosphere;Sealed tube;
Stage #2: with hydrogenchloride in methanol;water; pH=Ca. 3;Catalytic behavior;Reagent/catalyst;
Steps:
1; 3 Example 1 Asymmetric reduction coupling substrate expansion of aromatic imine
General procedure: In a clean and dry shlenk tube, add aryl aldehyde (0.2mmol, 1.0eq),The system was evacuated three times with nitrogen, protected by nitrogen, under nitrogen protection, added ammonia methanol solution (7.0M, 3.0mmol, 15.0eq), added solvent and distilled tetrahydrofuran (2mL), the system was sealed, the reaction was stirred at room temperature for 1h, under nitrogen , Add DB4 (0.11mmol, 0.55eq),Stir for 12-24h at room temperature (20-30°C). Rotate the system to remove excess ammonia gas,Add 1mL methanol, add hydrochloric acid (3.0M, 1mL) to adjust the pH to about 3,After washing with dichloromethane, the organic phase was concentrated and then slurried with n-hexane to recover the chiral diol diol5 (95% recovery rate).The aqueous phase is adjusted to pH 12 by adding sodium hydroxide solution and extracted with dichloromethane,The organic phases are combined, dried over anhydrous sodium sulfate, and the organic phase is spin-dried to obtain the product.
References:
CN111440205,2020,A Location in patent:Paragraph 0130-0135; 0248-0252

100-52-7
951 suppliers
$20.37/250g

35132-20-8
361 suppliers
$7.00/1g
![Spiro[(2-exo)-7-azabicyclo[2.2.1]hept-5-ene-2,2'-imidazolidine]-7-carboxylic acid, 4',5'-diphenyl-, 1,1-dimethylethyl ester, (1α,2β,4α,4'R,5'R)-](/CAS/20210305/GIF/636999-35-4.gif)
![7-Azabicyclo[2.2.1]hept-2-ene-7-carboxylic acid, 5-oxo-, 1,1-dimethylethyl ester, (1S,4S)-](/CAS/20210111/GIF/635298-68-9.gif)
![Spiro[(2-exo)-7-azabicyclo[2.2.1]hept-5-ene-2,2'-imidazolidine]-7-carboxylic acid, 4',5'-diphenyl-, 1,1-dimethylethyl ester, (1α,2β,4α,4'R,5'R)-](/CAS/20210305/GIF/635298-66-7.gif)
![tert-Butyl (1R,4R)-5-oxo-7-azabicyclo[2.2.1]hept-2-ene-7-carboxylate](/CAS/20210305/GIF/635298-67-8.gif)