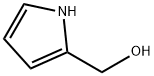
(1H-PYRROL-2-YL)-METHANOL synthesis
- Product Name:(1H-PYRROL-2-YL)-METHANOL
- CAS Number:27472-36-2
- Molecular formula:C5H7NO
- Molecular Weight:97.12
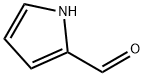
1003-29-8
438 suppliers
$10.00/1g

27472-36-2
31 suppliers
$320.00/1g
Yield:27472-36-2 96%
Reaction Conditions:
with hydrogen in isopropyl alcohol at 110; under 22502.3 Torr; for 3 h;Autoclave;
Steps:
2.3. Hydrogenation reactions
General procedure: FFR (99%, Sigma-Aldrich) 1 mL and various amounts of thenickel catalysts were added to 30 mL of a solvent (methanol(99.8%, Duksan), ethanol, IPA, or methyl isobutyl ketone (MIBK;99.5%, TCI)). Raney Ni (Ni 92.5%, TCI) was washed with ethanoland distilled water twice each and dried in a vacuum oven at50 °C before use. The mixed solution was placed in a 100 mL Teflonliner with a magnetic stir-bar and sealed in a stainless steel autoclave.Then the reactor was purged with H2 three times to excludeother gases. The autoclave was heated to 110 °C at which temperaturethe hydrogenation reaction was performed under 30 bar H2and stirring at 700 rpm. After the reaction, the autoclave wascooled, and the solution was subsequently centrifuged at11,000 rpm for 10 min to separate the liquid-phase products fromthe catalyst. For the recycling tests, 1 mL of FFR and 30 mL of IPAwere added to the centrifuged Ni catalyst without any furthertreatment. The products were analyzed by a gas chromatograph(GC; YL 6100) equipped with a capillary column (DB-624, AgilentTechnologies, 30 m 0.53 mm 3.00 lm) and flame ionizationdetector (FID).The selective hydrogenation of various unsaturated aldehydesand ketones was performed according to the following protocol.The reactant 1 mL (3-cyclohexene-1-carboxaldehyde (97%,Sigma-Aldrich), 5-hydroxymethyl-2-furaldehyde (99%, Sigma-Aldrich), 5-methylfurfural (99%, Sigma-Aldrich), 2-thiophenecarboxaldehyde (98%, Sigma-Aldrich), pyrrole-2-carboxaldehyde (98%, Sigma-Aldrich), 3-furancarboxaldehyde(P97%, Sigma-Aldrich), 2-furyl methyl ketone (99%, Sigma-Aldrich), acetophenone (99%, Sigma-Aldrich), cinnamaldehyde(99%, Sigma-Aldrich), or trans-2-hexen-1-al (98%, Sigma-Aldrich))was added to 30 mL of IPA. The 6.8 nm Ni nanoparticle catalyst(20 mg) was charged to the reactor. The hydrogenation reactionswere then performed according to the FFR hydrogenation protocol,except the reaction temperature was 100 °C for 3-cyclohexene-1-carboxaldehyde.
References:
Jeong, Hojin;Kim, Chanyeon;Yang, Sungeun;Lee, Hyunjoo [Journal of Catalysis,2016,vol. 344,p. 609 - 615]
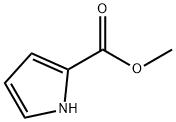
1193-62-0
224 suppliers
$5.00/1g

27472-36-2
31 suppliers
$320.00/1g