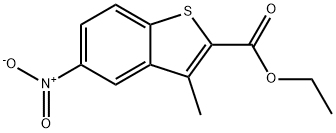
ETHYL 3-METHYL-5-NITROBENZO[B]THIOPHENE-2-CARBOXYLATE synthesis
- Product Name:ETHYL 3-METHYL-5-NITROBENZO[B]THIOPHENE-2-CARBOXYLATE
- CAS Number:17514-60-2
- Molecular formula:C12H11NO4S
- Molecular Weight:265.29
23082-50-0
146 suppliers
$9.00/250mg

623-51-8
232 suppliers
$7.00/25g
![ETHYL 3-METHYL-5-NITROBENZO[B]THIOPHENE-2-CARBOXYLATE](/CAS/GIF/17514-60-2.gif)
17514-60-2
21 suppliers
inquiry
Yield:17514-60-2 99%
Reaction Conditions:
with potassium carbonate in ethanol at 20;Reflux;
Steps:
35 4.1.35 Ethyl 3-methyl-5-nitrobenzo[b]thiophene-2-carboxylate (10)
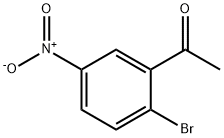
Ethyl 2-mercaptoacetate (8.21 mL, 7.52 mmol) was added into a solution of 1-(2-chloro-5-nitrophenyl)ethanone (1.0 g, 5.01 mmol) in dry ethanol (25 mL), followed by addition of K2CO3 (830.93 mg, 6.01 mmol) with stirring overnight at room temperature. The reaction was monitored by TLC (PE:EA = 10:1).Then the mixture solution was poured into 100 mL water with stirring, and the precipitate was filtered and dried to afford 10 as a solid of green. (1.3 g, 99% yield). m.p. 164.0-166.1 °C. 1H NMR (300 MHz, CDCl3) δ 8.76 (d, J = 1.95 Hz 1H), 8.32 (dd, J = 8.92, 2.16 Hz, 1H), 7.96 (d, J = 8.90, 1H), 4.44 (q, J = 7.15 Hz, 2H), 2.86 (s, 3H), 1.45 (t, J = 7.13 Hz, 3H). HRMS(ESI): calcd, for C12H12NO4S [M+H]+ 266.0485, found 266.0482.
References:
Xu, Xiao-li;Yang, Ying-rui;Mo, Xiao-fei;Wei, Jin-lian;Zhang, Xiao-jin;You, Qi-dong [European Journal of Medicinal Chemistry,2017,vol. 137,p. 45 - 62]